Medical device translation refers to the process of translating various types of documentation related to medical devices.
This includes the translation of medical documents, such as user manuals, instructions for use (IFU), labels, packaging materials, software interfaces, regulatory documents, various marketing materials, and many others.
Due to the technical nature of these materials, medical device translations require specialized knowledge and expertise to achieve accuracy, clarity, and compliance with local and international regulatory requirements.
The end goal of this field of translation is to offer users, both patients and healthcare professionals, equal access and effective understanding of medical device documentation.
As one would assume, these requirements might be quite challenging to fulfil. Indeed, one needs to have a more in-depth understanding of the medical device translation industry to make the right choices.
In the following blog, we’ll help you gain a detailed overview of the importance and need of medical device translation as well as some of the best practices which you can implement when looking for and working with a medical device translation provider.
Are You Looking for professional Medical Device Translations?
Milestone works with native translators with domain expertise to accurately translate your medical device documents in 70+ languages. We provide translation certificates accepted by authorities across the globe.
Importance of accurate medical device translations
Accurate medical device translations are crucial for several reasons:
Ensuring patient safety: Inaccurate medical device translations can lead to misinterpretations of the instructions. This in turn can potentially jeopardize patient safety and health.
Guaranteeing medical professionals’ understanding: When working with medical devices, healthcare professionals rely to a great extent on accurate translations to properly utilize them. Their understanding in turn influences their assessment of the patient’s health and the optimal treatment for their conditions.
Offering better user experience: Accurate medical device translation facilitates a better user experience since all instructions, labels, and user manuals are provided in the user’s native language. This makes it easier for healthcare professionals and patients to use the device effectively.
Mitigating risks: Inaccurate translations can potentially result in adverse events. Thorough and accurate medical device translation processes would mitigate such risks, protecting the patient’s health as well as the medical professionals and the manufacturer’s reputation.
Also read: Pharmaceutical Translation : Importance Challenges & Best Practices
Ensuring regulatory approval: Translated documentation is often a requirement for the approval process for medical devices. Thus, accurate medical device translations would facilitate smooth and fast regulatory approval.
Ensuring legal compliance: Local authorities in many different countries have strict regulations regarding medical device labeling and documentation. Accurate medical device translations in turn ensure compliance with these regulations.
Enhancing professional reputation: High-quality translations are the best way to demonstrate a manufacturer’s commitment to professionalism and customer care. Providing all documentation and materials in the native language of professionals and patients would inevitably enhance their reputation in the global healthcare industry.
Enabling standardization: Consistent and accurate medical device translations contribute to standardization across international markets. This in turn contributes to reducing confusion and improving interoperability of medical devices across different markets.
Also read: ICF Translation Importance Requirements & Best Practices
Also read: Role Of Healthcare Translation In The Healthcare Industry
Enabling market access: To successfully enter international markets, medical device manufacturers must provide documentation in the local languages. Accurate translations in turn facilitate market access and new expansion opportunities across various international markets.
Overall, accurate medical device translations offer several benefits which in turn highlight its importance in the global medical device industry.
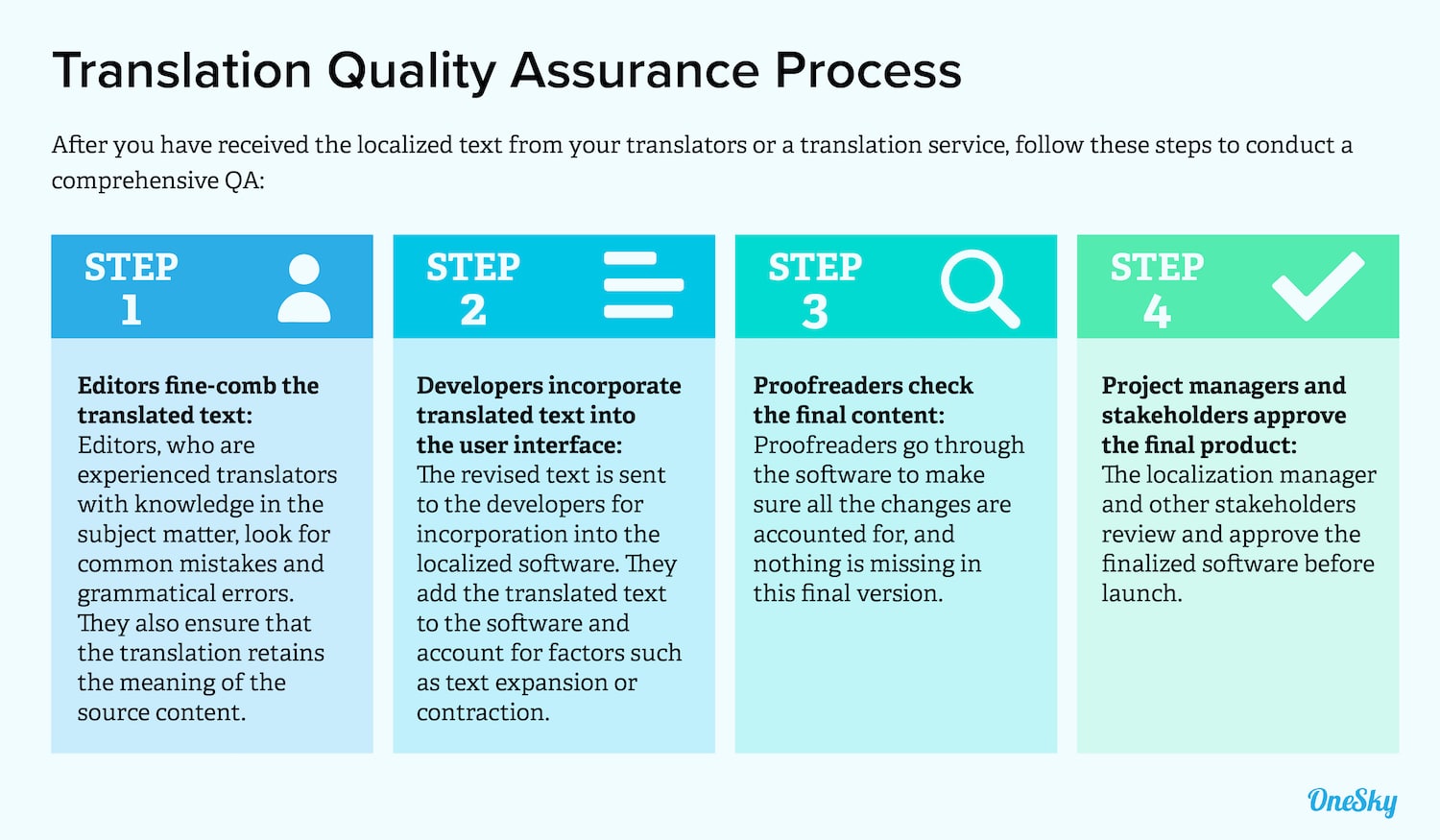
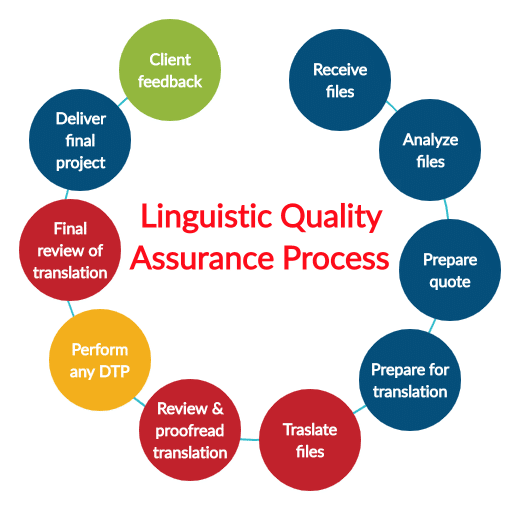
Need for a strong quality assurance process
Effective quality assurance processes are essential for the medical device translation industry to uphold the highest standards of accuracy, clarity, and regulatory compliance in translated documentation.
Given the critical nature of medical device documentation and materials, even minor errors or inconsistencies in translation can have dire consequences, ranging from patient harm to regulatory penalties, or legal liabilities.
Quality assurance processes help all stakeholders mitigate such risks by ensuring that translations undergo thorough review, validation, and testing to verify accuracy, linguistic consistency, and cultural appropriateness.
Also read: 5 Ways For Quality Assurance In Medical Document Translation
By implementing robust quality assurance measures, medical device translation can enhance patient safety, maintain regulatory compliance, ensure brand reputation, and foster trust among users and regulatory authorities in global markets.
Best practices for effective quality assurance in medical device translations
To ensure an effective quality assurance process, there are some best practices one can utilize to achieve high-quality, accurate medical device translation.
Qualifications of the translators: Always look into the qualifications of the translators working on the medical device translation project.
To ensure accuracy and regulatory compliance, professional translators receive specialized education and training to gain the required expertise to fulfill the requirements.
Translation process and methodologies: To fulfill the criteria of accurate medical device translations, language service providers must utilize certain translation processes and methodologies. When choosing an LSP, always inquire about these practices to ensure high-quality end translations.
Review by Subject Matter Experts: To mitigate the risk of any ambiguities or errors in the end translations, the final medical device translations should be reviewed by subject matter experts. Only subject matter experts can provide the knowledge and expertise in a specific subject to ensure quality end output.
Quality control checks: Every translation field, including medical device translation, has several quality control check procedures that ensure accuracy and precision. These procedures may include terminology consistency, accuracy verification, grammar and syntax, layout and formatting, cultural sensitivity, adaptation of numbers and units, feedback, and others.
Compliance with regulatory requirements: Adapting the medical device translations to the local regulatory requirements of your target markets is a must. Researching and creating a checklist of these requirements is essential for quality assurance in medical device translation.
Documentation and record keeping: Medical device translation is a project in progress. Regulations might change or new information might be added to old translations to accommodate professionals or authorities.
Thus, keeping a record of all translations, checklists, and other translation materials is crucial to ensure an efficient long-term translation process.
Also read: The Right Way To Do Medical Translation
A Comprehensive Guide To EU MDR Language Requirements
A free and comprehensive guide to help you seamlessly navigate the new language requirements as per the European Medical Device Regulation.
How to choose the best translation agency for accurate medical device translations?
Choosing the best translation agency for accurate medical device translations requires careful consideration of several factors, such as:
Evaluate expertise and experience: Look for translation agencies that have experience and relevant certifications (e.g. ISO 17100) in medical translation services. Also, consider their experience and expertise in working with medical terminology and medical requirements related to specific types of medical devices.
Assess QA processes: Inquire about the quality assurance processes for medical translations that the language service provider utilizes. Ensure they have robust procedures in place, such as multiple rounds of review by subject matter experts as well as linguistic validation, and compliance with regulatory standards.
Verify translators’ qualifications: Verify the qualifications and expertise of the translators who are assigned to your project. Ideally, translators should have a background in the following fields: medicine, biomedical engineering, or a related medical field. Of course, proficiency in both source and target languages is a must for translators in any field.
Consider regulatory compliance: Confirm that the translation agency is aware of and adheres to relevant regulatory requirements for medical device translation in your target markets. Always ask if they can provide certification or documentation to support compliance with regulatory standards. What’s more, you should always request samples and references of previous translation projects they have worked on.
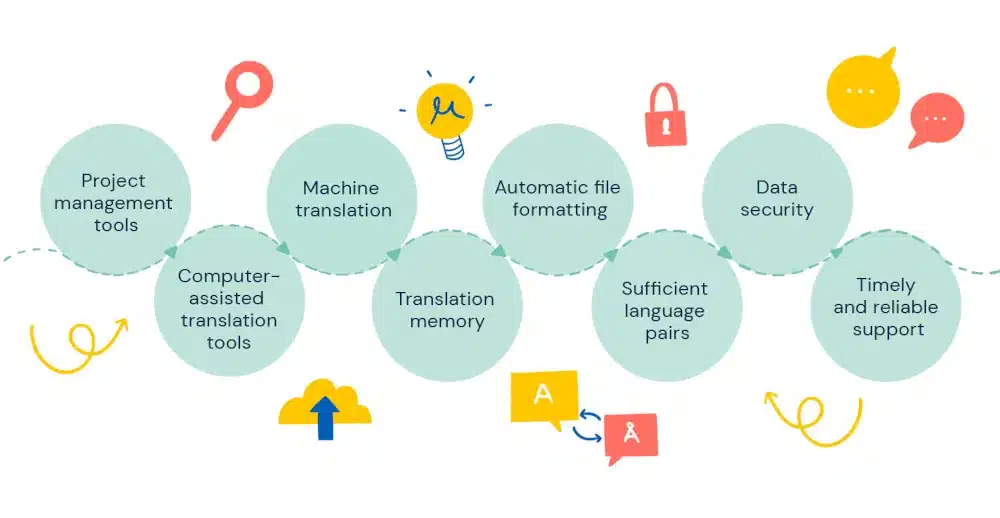
Evaluate technology and tools: Assess the technology and tools that the language service provider uses. Tools can significantly streamline processes. Thus, always look for agencies that leverage tools, such as Translation Memory (TM) tools and terminology databases, translation management systems, etc., tec. which ensures consistency and efficiency.
Consider cost and timeline: Always be open about your expectations regarding the budget and timeline of your translation project. While cost is important, prioritize quality and accuracy over price to avoid potential risks and liabilities associated with poor translations.
When looking for a translation agency for your medical device translation project, it is always helpful to look into and evaluate these factors. In this way, you can ensure that your translation project will run smoothly without any last-minute, unpleasant surprises.
Also read: Medical Translation Agency : How To Choose The Right One?
Conclusion
Medical device translation requires a high level of precision. This in turn can only be achieved through well-trained and experienced linguists and robust quality assurance procedures in place.
Thus, finding the right translation agency for your medical device translation project might be challenging.
However, having a good understanding of this field of translation while also being well-acquainted with the relevant qualifications and regulations would help you make the right choice for you and your translation project!
Are You Looking for professional Medical Device Translations?
Milestone works with native translators with domain expertise to accurately translate your medical device documents in 70+ languages. We provide translation certificates accepted by authorities across the globe.