Instructions For Use or IFUs serve as essential documents that provide important information about the proper use of products, devices, and equipment. In certain fields such as medical and medical devices, IFUs are not only informative but also legally required.
Regulatory bodies such as the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA), and the European Medical Device Regulation (EU MDR) enforce strict guidelines on IFUs to ensure the safety and effectiveness of medical products.
These regulations require IFUs to be clear, concise, and accurate, and to include all relevant information.
In this blog, we will explore the requirements for Instructions for Use (IFU) in the medical device industry, including IFU translation and how to approach it.
Are you Looking for Certified IFU translation services?
Milestone works with native translators with domain expertise to accurately translate your IFUs in 70+ languages. We provide translation certificates accepted by authorities across the globe.
What is Instructions For Use (IFU)?
Instructions For Use is a set of guidelines or directions provided with a product to inform users about its proper and safe use.
It generally contains all the details regarding the operation, assembly/installation, maintenance/troubleshooting, and disposal of the product to educate and ensure that users clearly understand the usage of the product.
IFUs are provided with various products such as electronic devices, medical devices, pharmaceuticals, surgical instruments, and more.
Instructions For Use of Medical Devices
In the medical device industry, IFUs are of heightened significance. The IFUs and user manuals for medical devices must meet the requirements mentioned in the applicable medical device regulations mentioned in the applicable medical device regulations by regulatory bodies such as FDA, EU, PMDA, CFDA, and others.
The medical device IFU must provide clear and detailed instructions for the safe and appropriate use of the device, which generally includes:
- The intended purpose of the device
- Description of the device and any other accessories or necessary components
- Warnings and precautions to be taken when using the device
- Instructions for proper maintenance, cleaning, and disposal
- Guidelines to deal with in case of errors and malfunctions
- Contact information for the manufacturer in case of queries, complaints, or adverse events
- Other necessary information for the safe use of the device
Download our free guide: Medical Device Translation Requirements As Per EU MDR
Medical Device IFU Translation
Medical Device IFU translation is the translation of medical device IFUs, or accompanying manuals, guidelines, or instructions,
These translations ensure that medical professionals, patients, and users worldwide can use medical devices safely and appropriately, adhere to safety procedures, and understand crucial information about their maintenance, operation, and possible risks.
Also read: Fastest Growing Medical Device Markets & Need For Translation
Importance of IFU Translations
Medical Device IFU translation holds particular importance due to the critical nature of medical devices and the potential consequences of improper use or misunderstanding. Here are some specific reasons why IFU translation is crucial in the context of medical devices:
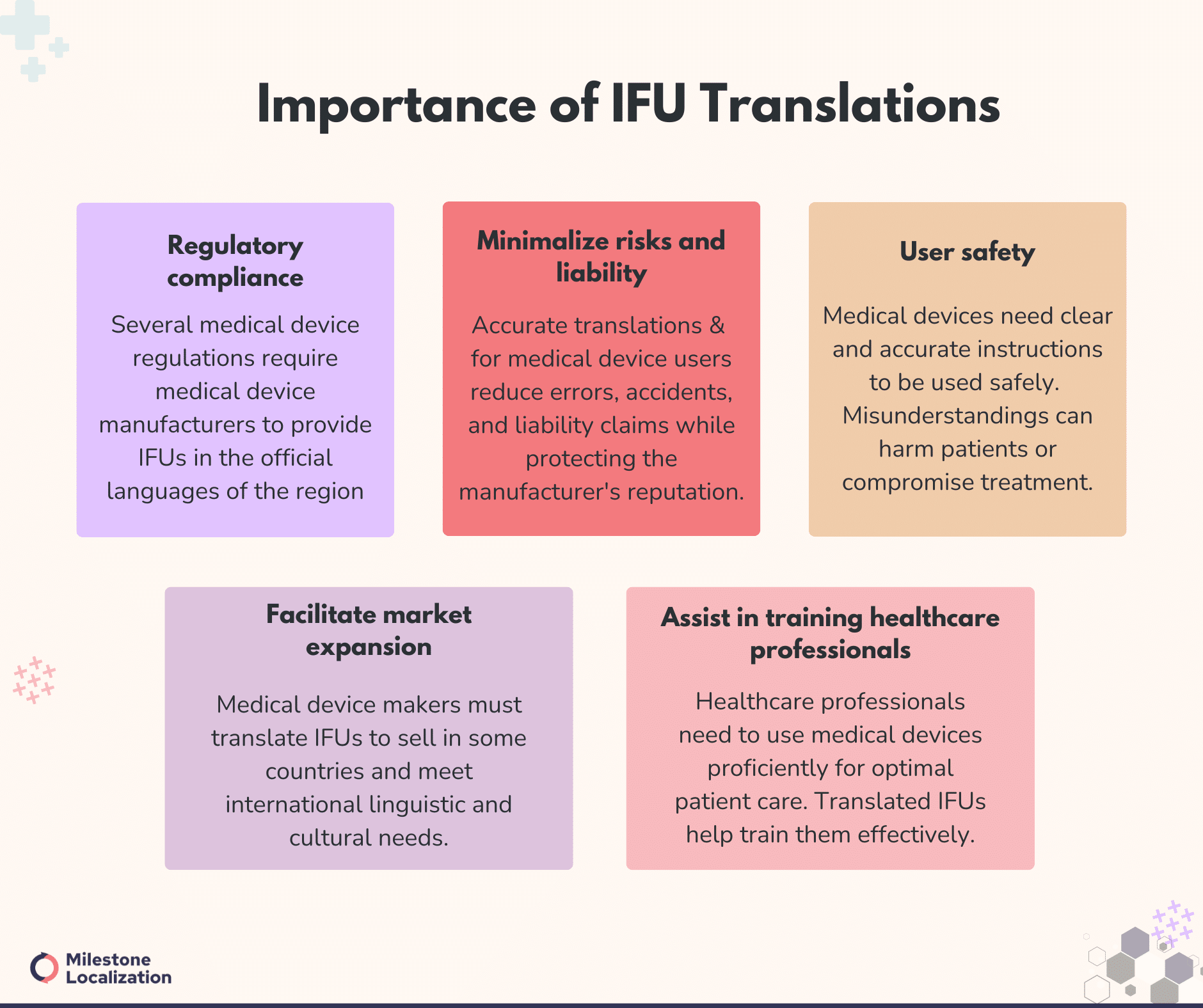
Regulatory compliance: Regulatory agencies such as the FDA in the United States and the EMA in Europe require medical device manufacturers to provide IFUs in the languages of the countries where the devices will be marketed or used.
Compliance with these regulations is mandatory and ensures that products meet quality and safety standards.
Also read: NAVIGATING PATENT TRANSLATION A Comprehensive Guide For Global Innovators
User safety: Medical devices are used in various healthcare settings, including hospitals, clinics, and home care.
For users to properly use medical devices safely and effectively, they must have access to clear, accurate information in a manner and language that is easy to understand.
Misunderstandings or errors in usage could lead to adverse events and harm the user or compromise the outcome of the device and treatment.
Minimalize risks and liability: Medical device manufacturers must provide clear and comprehensive instructions to users.
Accurately translated IFUs facilitate effective and clear communication with the interested user and help mitigate the risk of user errors, misuse, or accidents that could result in product liability claims, litigation, or damage to the manufacturer’s reputation.
Facilitate market expansion: Medical device manufacturers aiming to enter international markets must translate IFUs into the languages spoken in those markets.
By providing translated IFUs, manufacturers can meet the linguistic and cultural needs of diverse user populations, and expand their market reach.
Additionally, IFU translation is mandatory in various countries to market and sell medical devices in those regions.
Assist in training healthcare professionals: Healthcare professionals must be proficient in using medical devices to deliver optimal patient care.
Translated IFUs assist in training healthcare professionals and enable them to understand device specifications, operating procedures, maintenance requirements, and safety precautions effectively.
Also read: ICF Translation: Importance Requirements & Best Practices
Why Instructions for Use Translation Is Critical for Compliance and User Safety
Accurate Instructions for Use translation underpins regulatory adherence and prevents misuse of complex devices or products. By delivering instructions in each user’s preferred language, companies demonstrate respect for local laws while safeguarding end users from potential hazards.
In sectors like healthcare, even minor misunderstandings can pose serious risks, so thorough, clear IFU translations help maintain trust and minimize liabilities.
Medical Device Translation in Global Markets
The role of medical device translation continues to expand as manufacturers aim to reach new regions efficiently. Beyond meeting regulatory requirements, translating product information ensures doctors, nurses, and patients worldwide fully understand how to operate sophisticated medical equipment.
This localized approach supports better patient outcomes, reduces operational errors, and fosters a reliable brand reputation in the competitive global healthcare market.
IFU Language Requirements as per EU MDR
As per the regulations of EU MDR and EU IVDR, it is mandatory for the manufacturer to provide all information related to medical devices in the official languages of the European Union. However, each EU Member State reserves the right to decide which language version(s) of this information they will accept for their national market.
Accordingly, irrespective of whether the IFU is available in traditional paper form or electronic form, it has to be available in the official languages of the EU where the device is being sold.
The EU MDR/IVDR focuses on the below-mentioned translation requirements:
Readability: The IFUs have to be written in a way that is easily understood by the intended user. This means that the level of the language should be adjusted according to the understanding level of the intended user.
User can be a healthcare professional as well as a layperson without formal education in the relevant field of medicine or healthcare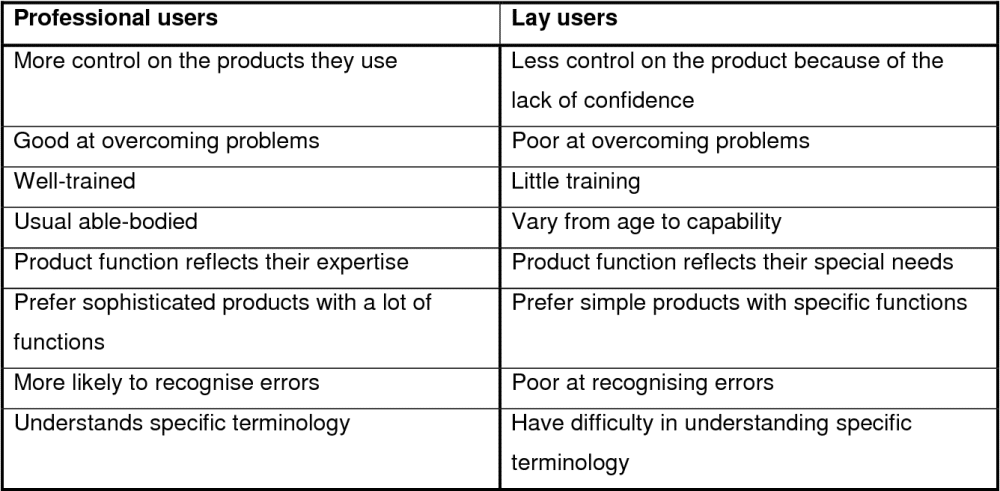
Translation into official EU languages: Each member state of the European Union is in charge of designating the official EU language or languages for the IFU included with a device sold in that state.
Furthermore, if the manufacturer maintains a website, it must provide and maintain current information on safety and performance relevant to the user or any other individual, as well as the information required to identify the device and its manufacturer.
According to Article 10(11), this information must be in all the language versions accepted in the national markets where the device is made available to the user or patient.
IFU translations need to be completed earlier in the workflow according to the new EU MDR since they must be submitted to the relevant notified body as part of the technical documentation for conformity assessment.
All class IIb and class III medical devices must have IFUs. However, class I and class IIa medical devices are exempted if the devices can be used safely without instructions.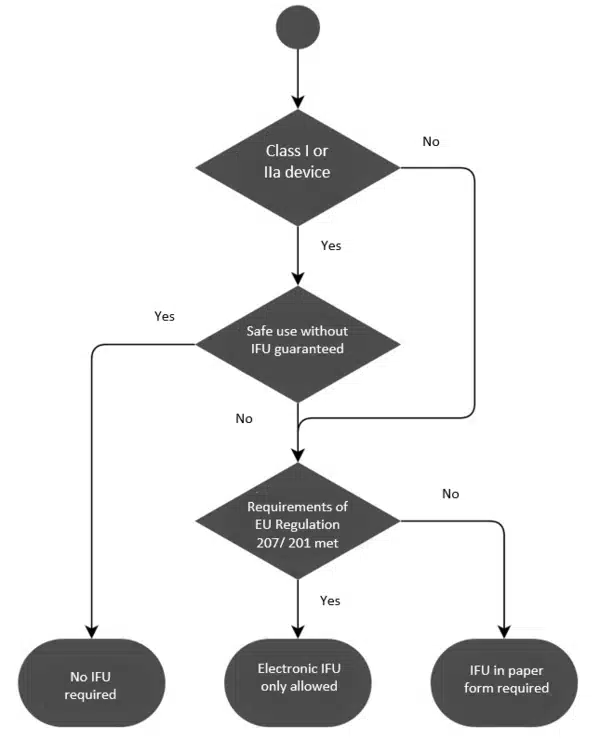
Also read: Pharmaceutical Translation Importance Challenges & Best Practices
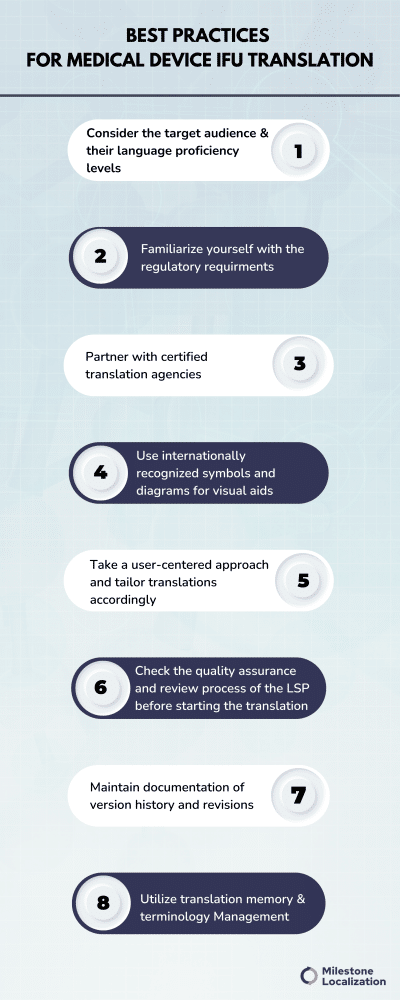
Best Practices For Medical Device IFU Translation
Also read: Role Of Healthcare Translation In The Healthcare Industry
Consider the target audience and their language proficiency levels
Various regulations such as EU MDR emphasize the readability of the IFUs which means, the IFUs should be in the level of language understood by the intended user. Therefore, it is important to research and analyze linguistic data to determine the languages the target audience speaks and their proficiency levels in those languages.
Consider regional variations in language usage and dialects within the target audience to tailor translations accordingly.
Regulatory language requirements
Familiarize yourself with the regulatory requirements for medical device documentation in the target markets, including language requirements specified by regulatory agencies such as FDA, EMA, and EU MDR.
Ensure that translations comply with the relevant regulations and standards such as
ISO 14971 for risk management in medical devices and ISO 15223-1 for symbols used in medical device labeling.
Work with certified translation agencies
Partner with certified translation agencies or language service providers with demonstrated expertise in medical device translation. Check for the agency’s certifications such as ISO 17100:2015 which is specific to translation services to ensure compliance with industry standards and regulatory requirements.
Language service providers work with a network of expert translators handpicked according to the project requirement. This ensures the quality and accuracy of the translation.
Visual aids, symbols, and diagrams
Use internationally recognized symbols (according to EN ISO 15223-1) and graphical representations white creating IFUs. This ensures that information is consistently conveyed across languages and regions.
Additionally, ensure visual aids are appropriate and accessible to users with diverse backgrounds and abilities.
User-focused approach
When it comes to medical, medical devices, healthcare, and pharmaceutical translations, the user may be a professional or a lay person. Therefore, take a user-centered approach and anticipate the context in which the device will be used such as clinical settings, home environments, or specialized care facilities, and tailor translations accordingly.
Incorporate feedback from user testing and usability studies to refine translations and improve user satisfaction.
Quality assurance and review process
Maintaining quality and consistency is significantly important while translating IFUs. It is important to check with the language service provider about their quality assurance process before starting the translation process.
Communicate your requirements and ensure that a comprehensive quality assurance is set in place to ensure the accuracy, consistency, and reliability of translated IFUs.
Verify if they conduct linguistic, technical, and regulatory reviews by qualified translators, subject matter experts, and regulatory specialists to identify and address any errors or inconsistencies.
Also read: 5 Ways For Quality Assurance In Medical Document Translation
Document version history and updates
It is important to maintain documentation of the version history and revisions to ensure compliance with regulatory requirements in IFU translation. So, it is important to establish robust version control procedures to manage changes and updates to the IFU content over time.
Utilize translation memory and terminology management
Utilize translation memory tools and terminology management systems to optimize translation efficiency and maintain consistency across translations.
Create and maintain a centralized repository of translated content, including reusable translation units and approved terminology to ensure consistency and accuracy.
Product Manual Translation and User Manual Translation for Diverse Products
Many industries depend on product manual translation and user manual translation to help end users understand setup, operation, and troubleshooting. From consumer electronics and home appliances to industrial machinery, localized manuals reduce user confusion and cut down on technical support requests.
Well-translated guides are not only a courtesy to international customers but also an effective way to uphold safety and performance standards.
Reliable IFU Translation Services
Milestone works with native translators with domain expertise to accurately translate your IFUs in 70+ languages. We provide translation certificates accepted by authorities across the globe.
IFU Translation Process
Translation of IFUs requires extreme attention to detail and a thorough understanding of the subject matter, technical terminology, and a comprehensive understanding of the context.
The IFU translation process at Milestone Localization is a robust procedure with a priority to uphold quality at every stage of the process:

Analysis and assessment of the project: Thorough analysis and understanding of the client’s project requirements, including the source language, target languages, timeline, and specific instructions. Based on this information, a custom project flow is prepared and tailored to the needs of the project.
Terminology Management and Glossary Creation: Consistent terminology is crucial in IFU translations. Therefore, a comprehensive glossary and terminology list of industry-specific terms, important technical terms, and any specific instructions are meticulously prepared to ensure consistency across all translations.
For instance, consider the word ‘site’. In everyday usage, it normally refers to a plot of land. However, its interpretation in the medical industry differs significantly. In the context of IFUs, ‘site’ specifically refers to the operational area where a particular activity or procedure is conducted.
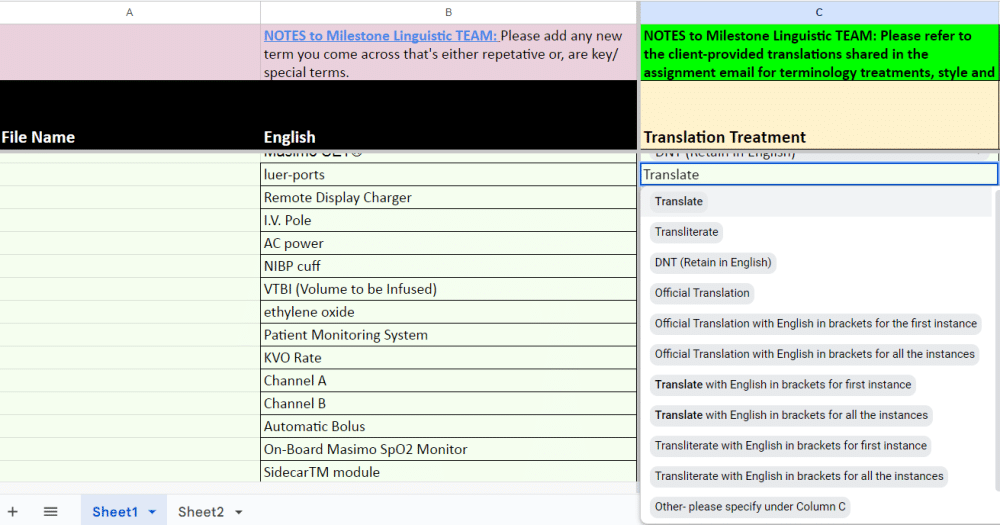
Created by Milestone Localization For A Client
It also involves identifying terms with an existing official translation specific to a domain and language. Various abbreviations, terms, and images have pre-existing official translations that have to be made use of while translating the content.
Initiating translation: Based on the language combinations required, the best-suited native translators who are fluent in both the source and target languages are assigned by the dedicated project manager. Milestone’s translators have extensive experience in the medical and healthcare industries, ensuring accurate and specialized translations.
Translation: Native translators with subject matter expertise and a minimum of 4 years of experience in translating medical device documents work on translating the documents under the guidance and instructions of the dedicated project manager.
Also read: The Right Way To Do Medical Translation
Proofreading: Every translated document is proofread by experienced proofreaders and subject matter experts to ensure linguistic excellence, adherence to guidelines, and error-free content.
Quality Assurance: Quality is at the core of our IFU translation process. The quality assurance team conducts meticulous checks to ensure adherence to industry standards, consistency, and accuracy.
Milestone performs detailed proofreading, formatting, and final review to deliver translations of the highest quality.
Feedback incorporation: The team actively engages with you to address any questions, concerns, or additional requirements you may have. Your feedback is incorporated into the translations to ensure they meet your expectations and specific project goals. Project managers closely monitor the progress of each IFU translation project to ensure timely delivery without compromising on quality.
Certification: Various regulatory bodies require translation certificates to confirm the accuracy and authenticity of translations. Milestone Localization provides translation certificates accepted and acknowledged by regulatory authorities worldwide.
Introducing Software Agreement Translation for EULAs and T&Cs
Modern devices and applications frequently require digital agreements, including EULAs (End-User License Agreements) or Terms & Conditions.Software agreement translation ensures these critical legal texts are enforceable and comprehensible in multiple regions.
Clear translations reduce misunderstandings, reinforce user trust, and help companies maintain compliance with local consumer protection laws.
Conclusion
Instructions For Use are an important document accompanying medical devices vital to not just the intended users but also necessary to comply with various rules and regulations.
Translating IFUs can be challenging considering the nuances and stringent quality requirements that are necessary. It requires meticulous care, attention, and subject matter expertise to ensure quality and accuracy.
However, by partnering with the right translation service provider and following the best practices, the process can be seamless.
Milestone Localization Life Sciences is a specialized unit of Milestone Localization dedicated to providing translation services to organizations in the life science industry.
Visit our website and get in touch with our team to discuss how our translation services can be the right fit for your language needs.
Also read: Medical Translation Agency: How To Choose The Right One?
Are You Looking for certfied IFU Translation Services?
Milestone works with native translators with domain expertise to accurately translate your IFUs in 70+ languages. We provide translation certificates accepted by authorities across the globe.
FAQS
What Is Instructions for Use (IFU) Translation and Why Is It Important?
Instructions for Use (IFU) translation refers to translating medical device manuals, pharmaceutical guidelines, and safety instructions to ensure compliance with international regulations. IFU translation is critical for:
- Regulatory approval by agencies such as the FDA, EMA, and MDR
- User safety and compliance for medical devices and equipment
- Global market expansion for manufacturers and healthcare companies
High-quality IFU translations reduce the risk of misuse, legal issues, and patient harm.
Why Is Medical Device Translation Essential for Global Compliance?
Medical device translation ensures that:
- Device instructions meet regulatory requirements in different countries
- Healthcare professionals and patients can safely use the product
Manufacturers avoid liability due to unclear instructions
Certified medical device translations help companies expand into international markets while ensuring safety and compliance.
What Is Product Manual Translation and Who Needs It?
Product manual translation involves translating instruction guides for electronic devices, industrial machinery, consumer goods, and medical products. Industries that require multilingual product manual translations include:
- Medical and healthcare device manufacturers
- Technology and electronics companies
- Automotive and engineering firms
Professional product manual translation services ensure that end users can properly operate and maintain products.
How Is User Manual Translation Different From General Translation?
User manual translation requires:
- Technical accuracy to avoid product misuse
- Compliance with industry-specific regulations
- Clear and concise language for international users
Unlike general translations, user manual translation ensures that product instructions are correctly interpreted by global customers.
What Are the Regulatory Requirements for IFU and Medical Device Translations?
IFU and medical device translations must comply with regulations such as:
- EU MDR (Medical Device Regulation) for European markets
- FDA guidelines for medical devices sold in the United States
- ISO 13485 and ISO 17100 for quality assurance in medical translations
Certified medical document translation services help companies meet these strict regulatory standards.
How Much Do IFU Translation Services Cost?
The cost of IFU translation depends on:
- Language pair (English to German, Chinese, French, etc.)
- Technical complexity (medical, pharmaceutical, industrial manuals)
Regulatory compliance requirements (certified vs. non-certified translations)
On average, rates range from $0.12 to $0.30 per word*, with certified translations priced higher due to legal and regulatory requirements.
Which Industries Benefit the Most from IFU and Medical Device Translations?
Several industries require IFU and medical device translations, including:
- Medical Device Manufacturers: Instructions for patient safety and regulatory approval
- Pharmaceutical Companies: Medication guides and usage instructions
- Technology & Electronics: User manuals for international consumers
- Industrial Equipment Manufacturers: Technical documentation for proper machinery operation
Using expert medical device translation services ensures compliance, accuracy, and patient safety.