Informed consent is a fundamental ethical principle in research that ensures that participants or the subjects of the study fully understand the nature of the study and voluntarily agree to participate.
It involves providing individuals with comprehensive information about the research study, including its purpose, procedures, potential risks, and benefits.
This blog post aims to explore the significance of Informed Consent Forms (ICF) and ICF translation requirements by various regulatory bodies. You will also have a comprehensive understanding of the best practices to ensure high-quality translation of informed consent forms.
Are you Looking for professional ICF translation services?
Milestone helps you accurately translate your Informed Consent forms, clinical trial documents, pharmaceutical documents, and more in 70+ languages.
Informed consent form and its importance
Informed consent forms play a critical role in various fields and contexts, ensuring that individuals make voluntary and informed decisions regarding their participation in specific research, activities, or treatments.
These forms are utilized in medical research, clinical treatments, behavioral studies, biomedical procedures, educational research, and other areas where participant consent is essential.
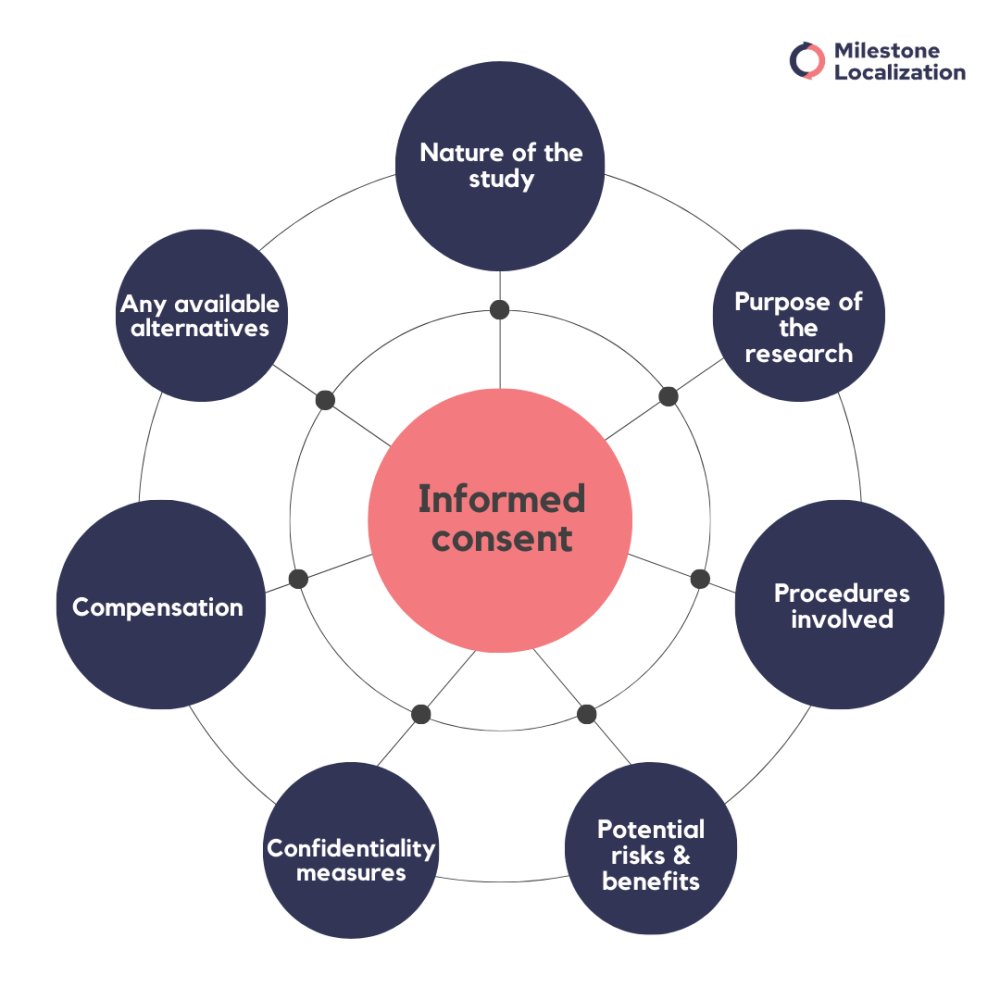
An informed consent form typically includes:
- Comprehensive details about the study, treatment, or procedure
- Key information, such as the purpose of the research
- Procedures involved
- Potential risks and benefits
- Confidentiality measures
- Compensation
- Any available alternatives
Additionally, the form may also outline the participant’s rights, including the right to withdraw from the study at any time without penalty.
By providing participants with this essential information, the informed consent form serves as a formal agreement between the participant and the researcher, ensuring that both parties understand and agree to the terms and conditions of participation.
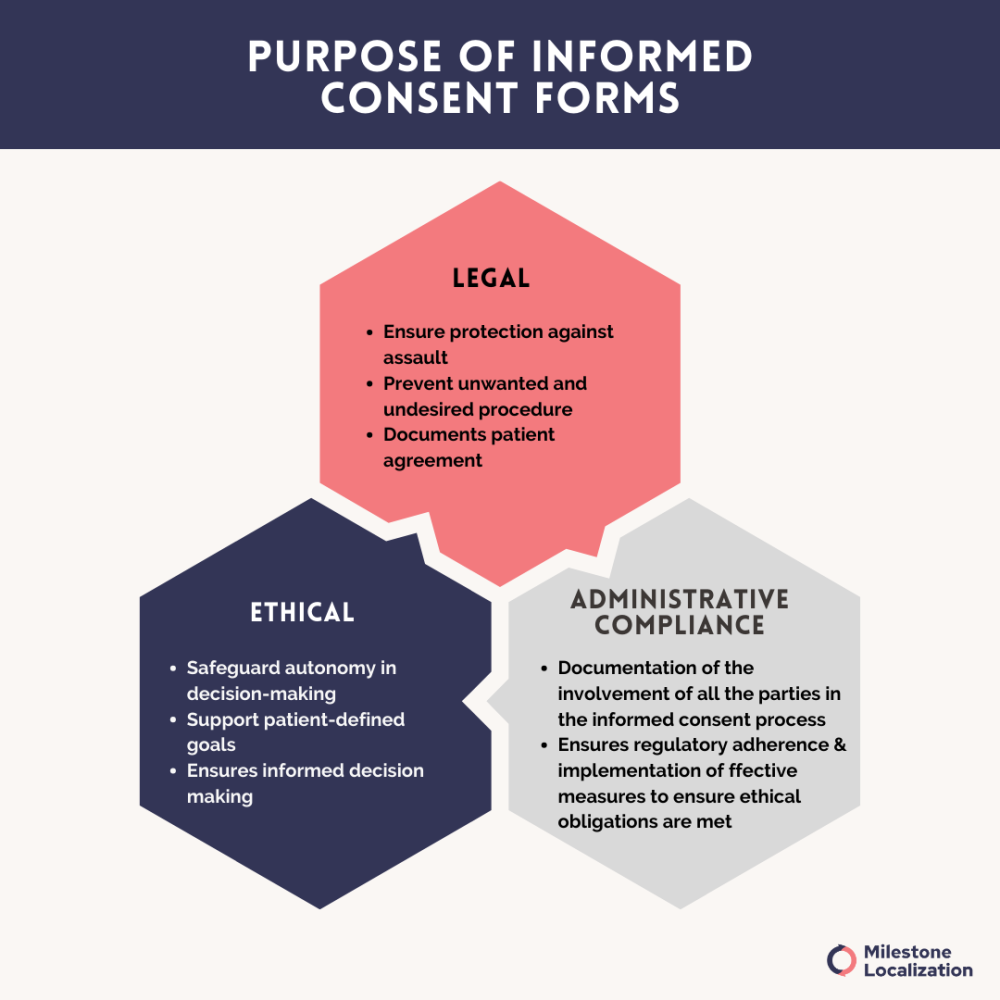
The primary objective of the informed consent process is to safeguard the rights, autonomy, and well-being of participants.
By signing the informed consent form, participants acknowledge that they have been adequately informed about the study and voluntarily agree to participate.
Even researchers in a medical school meticulously use the informed consent form in their medical research to ensure their research participants are well informed.
Responsibility for ensuring informed consent
In obtaining informed consent, multiple parties share the responsibility:
- The Institutional Review Board (IRB)
- Clinical investigator
- Research sponsor
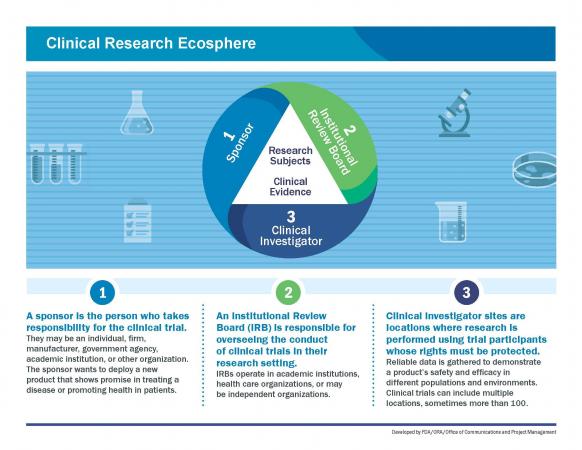
However, the primary responsibility for securing informed consent is of the clinical investigator or licensed physician investigator. It is the investigator’s responsibility to ensure the informed consent form is obtained, documented, and understood by the participant.
This responsibility extends regardless of any assistance provided during the consent process. The investigator bears the responsibility for adhering to ethical and regulatory standards in obtaining informed consent from study subjects.
Download our free guide : Medical Device Translation Requirements as per EU MDR
Why translate informed consent forms?
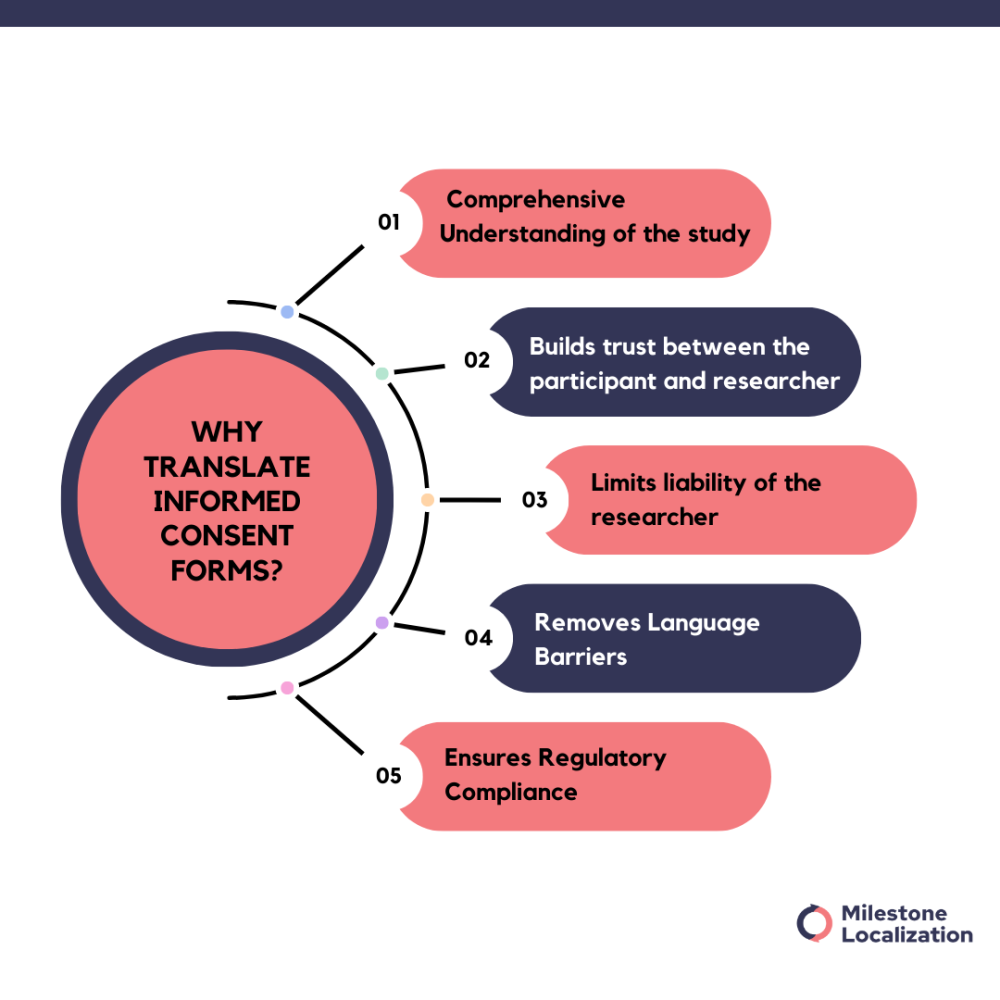
Translating informed consent forms is crucial because:
Comprehensive Understanding: It ensures that all participants fully understand the purpose, procedures, risks, and benefits of the study, enabling them to make informed decisions about participation.
Builds trust: Clear communication builds trust between researchers and participants, upholding ethical principles and fostering transparency.
Limits liability: Translated informed consent forms confirm the voluntary consent of the participant, reducing researchers’ liability and ensuring compliance with ethical and regulatory standards.
Language Barriers: Translation overcomes linguistic obstacles, facilitating effective communication with diverse populations and promoting inclusivity in research.
Regulatory Compliance: Many regulatory bodies require translated consent forms to uphold ethical research practices and protect participant rights.
Reliable and accurate ICF translation services
Milestone helps you accurately translate your Informed Consent forms, clinical trial documents, pharmaceutical documents, and more in 70+ languages.
When should I translate informed consent forms?
Informed consent forms should be translated in situations where language barriers may hamper participants from clearly understanding the content and making informed decisions.
Participants have the right to know and should be fully informed of the procedures, potential risks, and benefits of the study. More so when the potential risk of study is high. Therefore, the informed consent form should be tailored to meet the level of understanding of the participants.
Here are some key scenarios when translation is necessary:
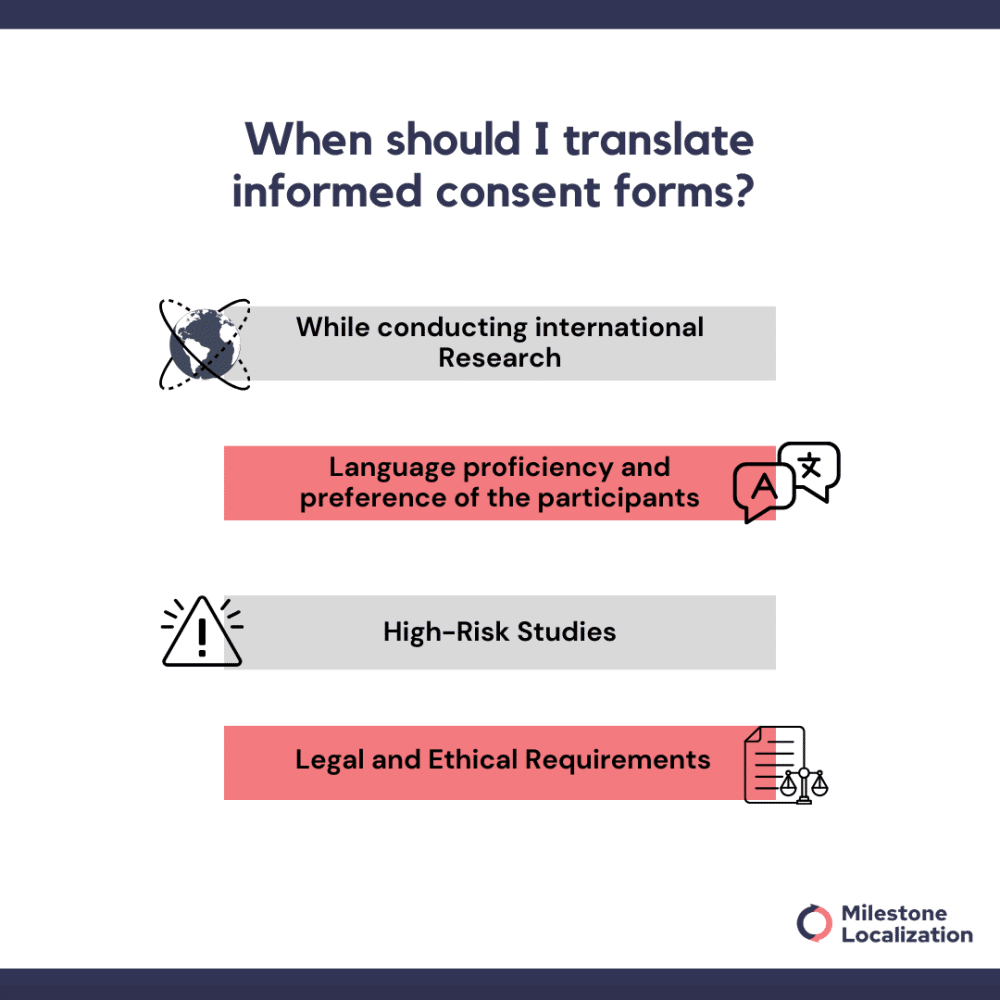
International Research: For studies involving participants from different countries or linguistic backgrounds, translating the informed consent form is essential to ensure all participants can comprehend the information provided.
Language proficiency and preference of the participants: If participants are not proficient in the language of the original consent form or express a preference for another language, providing a translated version of the form is necessary to facilitate their understanding.
High-Risk Studies: In research studies where the potential risks are significant, it is particularly important to ensure that participants fully grasp the procedures, risks, and benefits. Translating the consent form helps tailor the information to the participants’ understanding level.
Legal and Ethical Requirements: Some regions or institutions have legal or ethical requirements mandating that informed consent forms be provided in the participant’s native language. Compliance with these regulations is essential to upholding ethical standards and ensuring the validity of consent.
Translating informed consent forms is necessary to accommodate diverse participant populations, ensure comprehension of study information, and uphold legal and ethical standards in research. It is crucial to assess the linguistic needs of participants and provide translated materials accordingly to facilitate informed decision-making.
EU Requirements For Translating Informed Consent Forms
The new European Clinical Trial Regulations (CTR) went into effect on 31st January 2023, at which point all clinical trials are subject to EU CTR. According to the CTR, all the documents related to clinical trials are required to be translated into the language(s) of the participants.
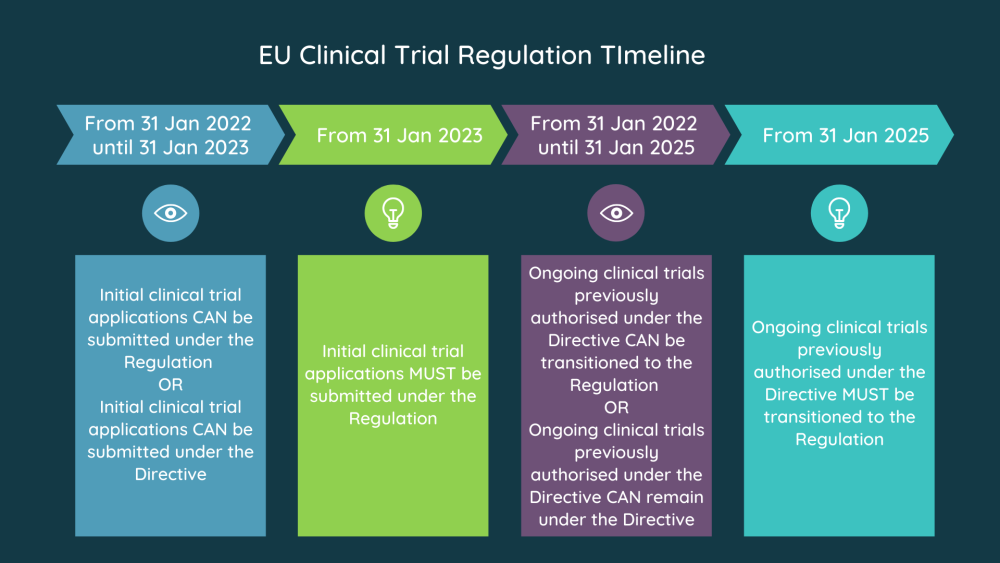
The regulation applies to all clinical trials conducted in the EU, irrespective of the location of the participants or sponsors. Furthermore, the sponsors must maintain records of all translations associated with the trial. The records must include details such as the name of the translator, the date of translation, and any changes made during the translation process. This ensures transparency and accountability in the translation process and facilitates regulatory compliance.
Additionally, all the documents that are to be submitted to the regulatory authorities in the EU must be translated into the official languages of the EU member states.
Also read: Role of Translation In The Healthcare Industry
Requirements for ICF translation in India
The Indian Drugs and Cosmetics Acts and Rules have laid down specific guidelines concerning the languages used in the Informed Consent Forms:
English and/or the vernacular language of the participant(s) should be used for the informed consent forms and the patient information sheet.
Although each institutional ethics committee has its own application form and clearance requirements, the basic requirements shared by all of the Indian ECs, as listed by the G-ICMR mention that informed consent documents (in English and local languages), including translation and back translation certificates, if applicable.
As stated in the 2019-CTRules and the G-ICMR, the ICF should be written in English and/or in a vernacular language that the participant is capable of understanding. Additionally, per the G-ICMR, the language in the ICF should be scientifically accurate, simple, and sensitive to the participant’s social and cultural background.
United States Requirements For Translating Informed Consent Forms
For research studies conducted under the jurisdiction of the US. Food and Drug Administration (FDA), the FDA regulations and ICH guidelines require that the information that is given to the subject or the representative shall be in a language understandable to them.
This means that any information, including the informed consent form, must be presented in the language and at a level that is understandable by the subject. This includes explanations of scientific, medical, and technical terms as well.
Additionally, an Electronic Informed Consent Form (eIC) should also contain all elements of the informed consent required by HHS and/or FDA regulations in a language and at a level that the potential subject or representative can comprehend. The information must be conveyed in a manner that minimizes the possibility of coercion or undue influence, thereby safeguarding the subject’s autonomy to participate in the study.
The FDA emphasizes the importance of accurate and understandable translations, acknowledging that linguistic and cultural differences can impact participants’ comprehension.
Translators must be well-versed in both the source and target languages, as well as the nuances of the respective cultures. Moreover, the translated ICF must undergo rigorous review and validation processes to ensure accuracy and consistency.
Also read: Medical Translation Agency: How To Choose The Right One?
Back translation for quality control in ICF translation
Back translation is a process where the translated content is translated back to the source language as a quality control measure to check the accuracy of the translations. A second translator with excellent command of both languages will translate the ICF from the target language back to the source language.
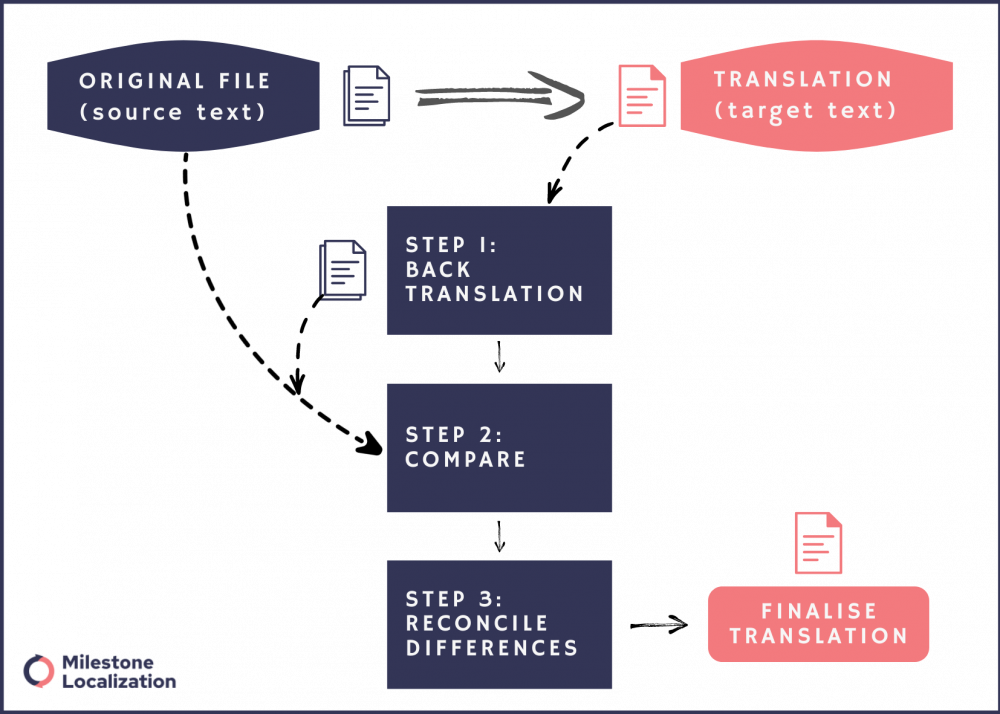
By comparing the source text with the back-translated version, discrepancies or errors in the translation can be identified and addressed. This method helps ensure that the translated content accurately reflects the intended meaning of the original text and maintains consistency across languages.
Additionally, back translation can be particularly useful in specialized fields such as medical or technical translation, where precision and clarity are significant.
Depending on the risk involved, the ICF may need to be back-translated, specifically when the risk involved is high. Further, to ensure informed consent for medical and pharmaceutical trials that pose a higher risk to the participants, additional quality assurance may be required by the Institutional Review Boards (IRB)
Also read: Back Translation – What Is It And Why Is It Important?
Overview on Clinical Trial & Patient Consent Translation
In high-stakes research, ICF translation and informed consent form translation are pivotal for building trust among study participants. Whether it’s a medical document translation for new drug trials or adapting patient consent translation for local languages, accuracy determines ethical compliance and participant safety.
Additionally, clinical trial translation extends beyond mere linguistics – it must capture cultural nuances so patients fully grasp potential risks and benefits. Investing in specialized translators reduces confusion, maintains regulatory alignment, and encourages willing participation, even in multinational trials.
Best Practices for Informed Consent Form Translation
The successful translation of ICFs requires a nuanced approach. Here are some best practices to ensure the efficacy of the process:

Start with a carefully prepared source document: It is vital to ensure that the source document is accurate and has gone through a quality assurance process to ensure it is free from errors. If not, the translated informed consent form would also be incorrect, and rectifying it would only be a waste of time, money, and resources.
Work with professional translators: Collaborate with experienced translators who specialize in medical and scientific language, ensuring the accuracy and clarity of the translated content. Various language service providers are specialized in providing translation services for the life sciences industry.
They work with a network of professional translators hand-picked to ensure they deliver quality services that are required for documents such as ICF, where there is no room for errors.
ISO 17100 Certification: It is advisable to get the ICF translations done from a language-certified provider who is ISO 17100 certified. This certification is specific to translation, and ISO certified agencies have robust processes in place to ensure quality is maintained at every stage of the translation process, resulting in accurate translations.
Create a glossary for key terms: Compile a list of key scientific, medical, and technical terms that are crucial for understanding the informed consent forms. A well-prepared glossary and term list help make the translation process more efficient and ensure accurate and consistent translations.
Communicate your requirements to the translator or LSP: The content of the Informed Consent Form should be at a level that is easily understood by the patient or the research participant. Therefore, it is necessary to clearly communicate the tone, readability level of the content, etc., before beginning the translation process.
Validation and Testing: Subject the translated ICF to rigorous validation and testing processes, involving members of the target population to assess comprehension and identify potential areas of confusion.
Document and Maintain Records: Maintain detailed records of the translation process, including the qualifications of translators, validation procedures, and any modifications made. This documentation may be crucial for regulatory compliance.
Also read: Pharmaceutical Translation: Importance, Challenges & Best Practices
Highlighting the Role of Professional Medical Document Experts
Opting for experts well-versed in medical document translation ensures that Informed Consent Forms, protocols, and participant instructions preserve their critical details.
Agencies employing subject-matter specialists and ISO 17100 workflows are better equipped to handle clinical trial translation challenges, from complicated terminology to patient-centered phrasing.
By combining linguistic precision with healthcare expertise, these professionals help sponsors and CROs safeguard participant rights while meeting global health standards.
In conclusion…
Informed consent forms are essential tools while conducting any research or clinical study. They help researchers explain their studies clearly and give participants all the information they need to decide if they want to take part.
Translating these forms is crucial to ensuring and confirming that the participants have understood all the nuances of the study. By focusing on translation and following best practices, researchers can build trust and ensure that their studies are ethical, inclusive, and compliant with various rules and regulations.
It is important to get ICF translations done by professional translators or language service providers specialized in such translations. Visit our website and get in touch with our team to discuss your translation requirements and see if we are the right fit for you.
Also read: Fastest Growing Medical Device Markets & Need For Translation
Are You Looking for professionals ICF translation services?
Milestone helps you accurately translate your Informed Consent forms, clinical trial documents, pharmaceutical documents, and more in 70+ languages.
FAQS
What Is ICF Translation and Why Is It Important?
ICF Translation refers to the translation of Informed Consent Forms (ICFs) used in clinical trials, medical research, and patient care. These documents must be accurately translated to ensure that patients fully understand the risks, benefits, and procedures involved in medical treatments. High-quality ICF translation services help:
- Ensure compliance with regulatory bodies such as the FDA, EMA, and IRBs
- Improve patient understanding across different languages
- Maintain consistency and accuracy in legally binding medical documents
What Are the Key Challenges in Informed Consent Form Translation?
Translating Informed Consent Forms (ICFs) requires expertise in medical terminology, legal compliance, and patient-friendly language. Key challenges include:
- Medical and legal accuracy – ICFs must be precise and compliant with regulations
- Cultural adaptation – Patients must understand medical procedures in a culturally appropriate way
- Clarity and readability – The translation should use simple and clear language to ensure informed patient decisions
Using certified medical translators ensures that ICF translations meet ethical and regulatory standards.
Why Is Medical Document Translation Essential for Clinical Trials?
Medical document translation is critical for clinical trials because it ensures that:
- Patients worldwide understand trial procedures and risks
- Regulatory submissions meet international compliance standards
Pharmaceutical and biotech companies can expand globally
Certified clinical trial translation services guarantee accuracy, compliance, and patient safety.
What Is Clinical Trial Translation and What Documents Require It?
Clinical trial translation involves translating documents needed for medical research, drug development, and patient communication. Essential documents include:
- Informed Consent Forms (ICFs)
- Patient information leaflets
- Regulatory submission documents
- Case report forms (CRFs)
- Clinical trial protocols
Ensuring precise and legally compliant translations is crucial for successful clinical trials and regulatory approvals.
How Is Patient Consent Translation Different From General Translation?
Patient consent translation requires:
- Clear and simple language for patient comprehension
- Medical and legal terminology expertise
Compliance with ethical guidelines for informed decision-making
Unlike general translations, ICF translation ensures that patients fully understand their rights and medical procedures before signing consent forms
How Much Do Professional ICF Translation Services Cost?
The cost of ICF translation depends on:
- Language pair (English to Spanish, French, Chinese, etc.)
- Document complexity (medical and legal accuracy required)
- Turnaround time (urgent translations may incur higher fees)
On average, ICF translation services range from $0.10 to $0.30 per word*, with certified translations costing more due to quality assurance and regulatory compliance.
Who Needs Professional ICF Translation Services?
Professional ICF translation services are essential for:
- Pharmaceutical and biotech companies conducting international clinical trials
- Hospitals and research institutions managing multilingual patient care
- Regulatory agencies requiring legally compliant translations
CROs (Contract Research Organizations) handling global studies
Accurate ICF and clinical trial translations help improve patient safety, regulatory approval, and research integrity.