The medical device industry is a fast-growing, highly regulated sector that serves patients and healthcare providers worldwide.
With a market valued at over $500 billion in 2024, the global market is expected to grow at a compound annual growth rate (CAGR) of around 6-7% over the next several years.
As companies expand their reach across different countries, clear and accurate communication becomes essential. Accurate translation of medical device documents is crucial not only for meeting strict regulatory requirements but also for protecting patient safety and ensuring healthcare professionals can use medical devices effectively.
In this blog, we’ll discuss the fastest-growing medical device markets, how to choose the right medical translation company, and the top medical device translation companies.
Top 10 Medical Device Translation Companies
1: Milestone Localization
Milestone Localization provides professional medical device translation services tailored to the unique needs of the healthcare industry worldwide. As an ISO 17100:2015, ISO 9001:2015, and ISO 13485:2016 certified agency, they uphold high standards of quality and security.
The ISO 13485:2016 certification reflects their specialized focus on medical device quality management, ensuring that all translation workflows align with strict industry regulations and best practices.
Milestone Localization works with 80+ medical device manufacturers and distributors around the world. They have published important reports about EU MDR language compliance along with several blogs for medical device companies.
Offering services in over 70 languages, they work with native translators and subject matter experts with extensive experience to guarantee accuracy and precision.
Their team is specialised in translation of IFUs, labels, manuals and UI for medical devices, with deep expertise on terminology, formatting and regulatory requirements.
Also read: Medical Device Translation Requirements As Per EU MDR
Get your Medical device documents translated by professional linguists in 70+ languages
2: RWS
RWS offers ISO 13485-certified translation, localization, and content management for medical device and in-vitro diagnostic documentation. They support MDR/IVDR compliance, technical authoring, regulatory affairs, and AI-enhanced processes to ensure accurate, timely global content delivery.
3: GTS (Global Translation Services)
 GTS specializes in certified medical device translations across 100+ languages, focusing on regulatory compliance and technical accuracy. Their ISO 17100 certification and experience with leading medical device companies ensure high-quality, reliable translations.
GTS specializes in certified medical device translations across 100+ languages, focusing on regulatory compliance and technical accuracy. Their ISO 17100 certification and experience with leading medical device companies ensure high-quality, reliable translations.
Their rigorous quality control processes ensure that the translated materials meet global regulatory standards to support safe, effective use of medical devices worldwide.
4: CCJK
CCJK provides medical device translation services in over 230 languages. With their ISO 9001, ISO 17100, and ISO 13485 certifications, they ensure the highest quality standards.
Their accurate, compliant translations help manufacturers meet global regulatory requirements and achieve smooth market access. With strong quality assurance process and dedicated project management, they deliver accurate, timely results.
5: Stepes
Stepes is a leading provider of medical device translation solutions, offering services in over 100+ languages worldwide. They combine industry knowledge with advanced technology to deliver translations that meet stringent regulatory demands.
Their team of specialized linguists ensures that all content is accurately translated to maintain the integrity and safety of medical devices across different markets.
6: Sesen
Sesen provides accurate, compliant medical device translation services for global market access. They translate IFUs, labeling, technical and regulatory documents, and embedded software with ISO-certified workflows, ensuring accuracy, regulatory alignment (FDA, MDR/IVDR), and patient safety across 150+ languages.
7: Okomeds

Based in Spain, Okomeds is a leading provider of medical device translation services, offering expertise in over 80 languages. Holding ISO 9001:2015 and ISO 17100:2015 certifications, they ensure translations meet the highest standards of accuracy and comply strictly with international regulatory frameworks.
Known for precision and reliability, they facilitate smooth regulatory approvals worldwide.
8: Mediqtrans
MediqTrans is a Singapore-based leader in medical device translation, providing specialized services in over 150 languages. With a network of more than 4,000 expert medical translators, they offer deep expertise in device terminology and global compliance requirements.
They help manufacturers deliver clear, accurate, and market-ready content that supports patient safety, regulatory approval, and successful international market entry.
9: Lionbridge
Lionbridge is an ISO 13485-certified medical device translation service company. They provide accurate and compliant translation of clinical content, labeling, and user materials.
Supporting all stages from R&D to post-market, they help manufacturers meet regulatory requirements and achieve efficient global market access.
10: Language Scientific
Language Scientific is a an ISO 9001 and ISO 17100 translation company that specializes in accurate medical device translations, by ensuring compliance with EU In-Vitro Diagnostic Regulation (IVDR) and other global standards.
They work with expert linguists and AI-optimized workflows to deliver accurate, high-quality translations in over 215 languages, supporting regulatory approval, patient safety, and global market access.
11: MarsTranslation
MarsTranslation offers professional medical device translation services to help manufacturers reach global markets. With a network of over 30,000 translators covering 230 languages, they ensure accurate, regulatory-compliant translations that resonate with target audiences.
Their expertise supports faster approvals and safe, effective use of medical devices worldwide
12: The Language Doctors Inc.
The Language Doctors offer ISO-certified medical device translation and localization services with over 25 years of experience.
They ensure precise, culturally adapted translations of technical documents, software interfaces, and marketing materials, helping manufacturers meet regulatory compliance and deliver safe, user-friendly medical devices worldwide.
The need for medical device translation
The need for medical device translation arises primarily from strict regulatory requirements and the critical importance of patient safety.
Regulatory bodies such as the European Union under the Medical Device Regulation (EU MDR) mandate that all labeling, instructions, and safety information be translated into the official languages of the countries where the devices are marketed to ensure clear understanding by users and healthcare professionals.
Accurate translation is essential not only to comply with these legal standards but also to prevent misuse, reduce risks, and facilitate effective and safe operation of medical devices across diverse linguistic markets.
Fastest-growing medical device markets
The global medical devices market has been growing rapidly in recent years, with new technologies and innovations driving growth in key regions worldwide.
According to reports, as of 2025, the global medical device market is valued at approximately $678.88 billion and is projected to grow steadily, reaching around $1,146.95 billion by 2034, with a compound annual growth rate (CAGR) of about 6% during this period
1: United States
The U.S. medical device market is valued at approximately $199 billion in 2025 and is projected to reach $315 billion by 2032 It is driven by strong regulatory frameworks, a culture of innovation, and widespread adoption of advanced technologies like wearables and surgical robotics.
2: China
China is the largest market for medical devices in the Asia-Pacific region with a high adoption rate for medical devices. The market is also expected to grow significantly due to the rise in demand for medical devices and improved healthcare infrastructure.
3: India
The Indian medical device market is expected to reach USD 61.55 billion by 2032. The market is driven by factors such as a rise in chronic diseases, healthcare expenditure and the improving healthcare infrastructure.
4: Japan
The Japanese medical device market is expected to reach USD 38.8 billion by 2032. The market is driven by factors such as a rise in demand for advanced medical devices and an increasing ageging population.
5: Germany
Germany boasts the largest medical device market in Europe. The country is known for its well-established healthcare system and has high adoption rates of advanced medical devices.
Also read: Fastest Growing Medical Device Markets & Need For Translation
How Can Medical Device Companies Benefit from Translation Services?
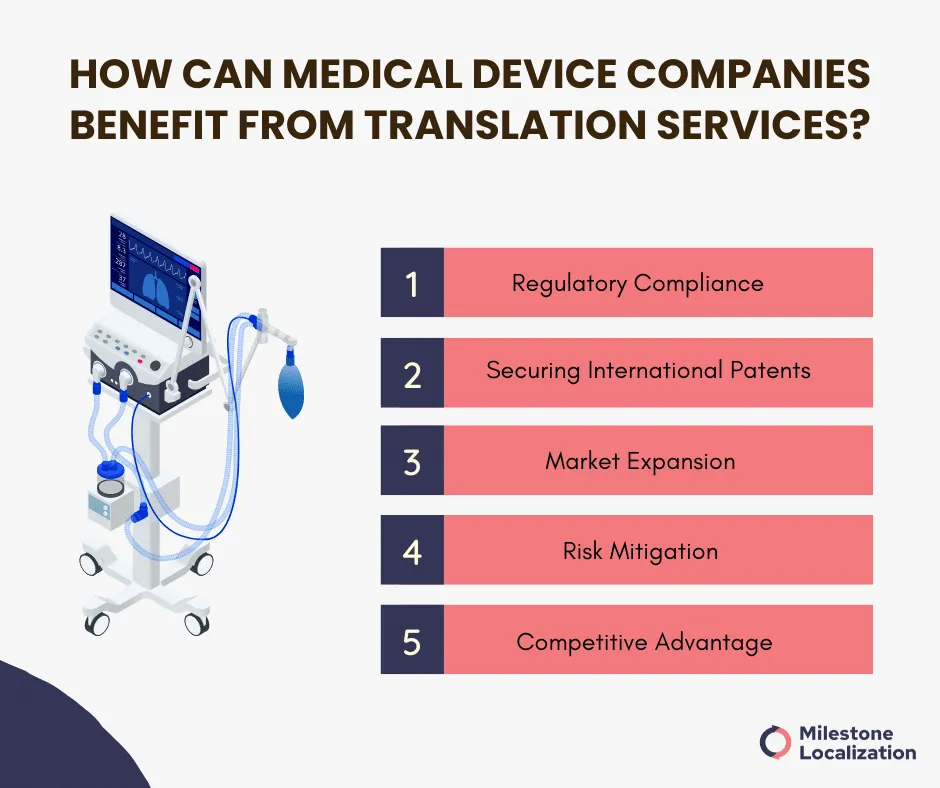
Regulatory Compliance
Medical device translation ensures compliance with regulations from authorities like the FDA, EU MDR, PMDA, CDSCO, and NMPA.
These regulations require medical device companies to provide accurate and complete documentation in the language of the target market. Failure to comply with these regulations can result in costly fines and delays in product approval.
So translation services play a vital role in preparing regulatory submissions for approval in foreign markets.
Securing International Patents
Translations help meet language requirements for international patent filings, thereby protecting intellectual property across multiple countries.
Market Expansion
Translating product materials and marketing content enables companies to reach new customers. Collaborations with medical marketing firms help tailor the message to local audiences, boosting market presence.
Risk Mitigation
Translation services can also help medical device companies to improve the quality of their products. By ensuring that all documentation is accurately translated, companies can reduce the risk of errors, misunderstandings, and misinterpretations.
Competitive Advantage
Translation services help companies meet regulatory requirements, engage global customers, and maintain exceptional quality. This strategic approach fuels growth, builds credibility, and sets them apart in a competitive market.
Also read: Quality Assurance In Medical Device Translations
How to choose the right translation partner for your medical device translation?
1: Evaluate expertise and experience
Look for translation agencies with ISO 13485 certification, which reflects their ability to meet quality management standards for medical devices. Also consider their experience with medical terminology and their understanding of regulatory requirements for your specific type of medical device.
2: Rigorous Quality Assurance
Choose a translation partner who has a robust Quality assurance process that includes linguistic review, technical validation, and expert input. This ensures translations are accurate, consistent, and compliant with the regulatory standards.
3: Regulatory Compliance
Look for a translation partner who understands regulatory requirements and provides full support in preparing and managing documentation. They should ensure all translations meet regulatory guidelines and formats, helping to streamline approvals and minimize non-compliance risks.
4: Language Capabilities
Evaluate the partner’s ability to support all the languages required for your target markets. They should have a broad network of qualified translators fluent in the necessary languages to ensure effective localization and market penetration.
5: Technological Capabilities
Choose a translation partner that utilizes advanced technological tools, including translation management systems and CAT (Computer-Assisted Translation) tools. These technologies help streamline workflows, maintain consistency, and integrate seamlessly with your existing systems.
6: Confidentiality and Data Security
Verify that your translation partner implements strict confidentiality and data protection measures to safeguard sensitive and proprietary information, which is critical when handling medical device documentation.
7: Certifications of the Language Service Provider
Verify that your Language Service Provider has the right certifications. Look for ISO 17100 (standards for professional translation processes), ISO 9001 (comprehensive quality management systems), and ISO 13485 (quality management specific to medical devices).
These certifications demonstrate the provider’s commitment to strict, standardized processes that ensure reliable quality and regulatory compliance.
Also read: Medical Translation Agency : How To Choose The Right One?
Conclusion
The medical device industry is experiencing rapid growth and global expansion, making accurate translation more important than ever. As manufacturers enter diverse international markets, clear and accurate translations are essential for meeting regulatory requirements, protecting patient safety, and ensuring effective communication.
So, choosing the right medical device translation company is key to success in today’s global healthcare market. These specialized services ensure that complex documentation complies with local regulations and resonates with target audiences, supporting international market entry, maintaining industry standards, and building strong, trusted connections with patients and healthcare professionals worldwide.
Are you looking for professional Medical device translation services?
FAQS ON Top Medical Device Translation Companies
What makes a medical device translation company a top choice?
Top companies have specialized expertise in medical and regulatory terminology, hold relevant certifications (like ISO 13485), and use native translators with subject matter expertise to ensure accuracy and compliance.
Which certifications are important for medical device translation providers?
ISO 17100, ISO 9001, and ISO 13485 certifications are key, demonstrating quality management, security, and specialized medical device industry standards compliance.
How do translation companies ensure regulatory compliance for medical devices?
They provide translations aligned with regulations like EU MDR, local language requirements, and ensure documents like IFUs, labels, and vigilance reports meet regulatory standards.
What types of documents do medical device translation companies typically handle?
Common documents include instructions for use (IFUs), labels, manuals, clinical trial documents, regulatory submissions, post-market surveillance reports, and marketing materials.
How many languages do top medical device translation providers usually support?
Leading providers support 70 to over 150 languages, covering all major EU official languages and additional global markets to ensure comprehensive localization.
Why is subject matter expertise important in medical device translation?
Because medical devices use technical and regulatory jargon, translators with medical backgrounds ensure precise terminology and reduce risks of errors that affect patient safety.
Can specialized medical device translation companies help with EU MDR compliance?
Yes, experienced companies understand EU MDR’s stringent language and documentation requirements and provide tailored services to help manufacturers meet all regulatory translation obligations.