The EU just set a hard deadline for EUDAMED.
Starting May 28, 2026, manufacturers must use EUDAMED for registration across four modules.
Translation becomes a critical requirement for market access.
Manufacturers must provide device information, instructions for use, and technical documentation in the official languages of each member state where devices are marketed. Without accurate translations meeting regulatory standards, manufacturers cannot register devices or maintain market access.
This blog covers language obligations across EU member states and how to plan your translation strategy to meet the May 2026 deadline.
What is EUDAMED?
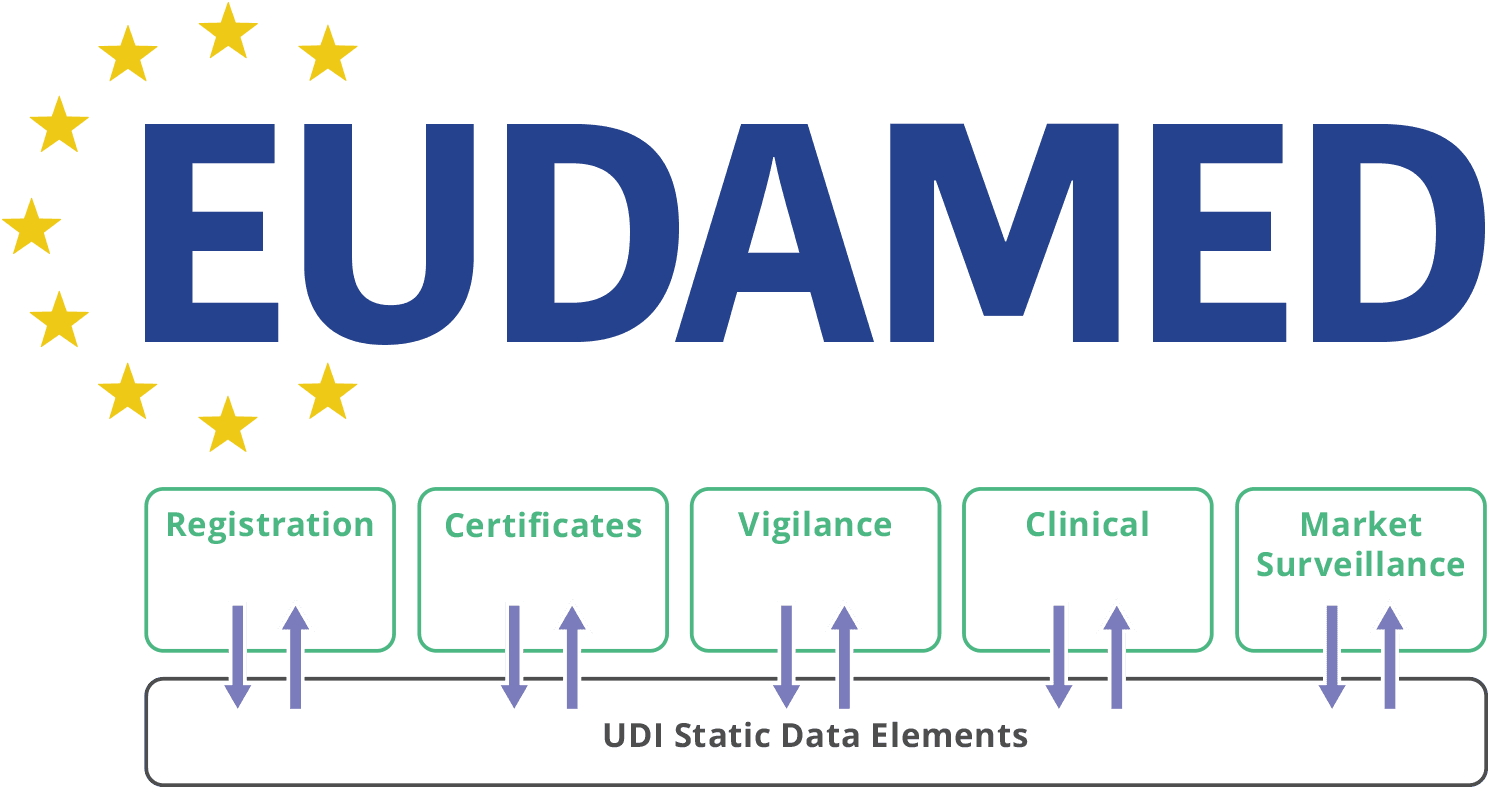
EUDAMED is the centralised IT system established under the Medical Devices Regulation (EU) 2017/745 (MDR) and In Vitro Diagnostic Regulation (EU) 2017/746 (IVDR). The database collects and shares information on medical devices and in vitro diagnostics across the European Union.
The Six Modules
EUDAMED consists of six modules:
- Actor Registration (ACT)
- UDI/Device Registration (UDI/DEV)
- Notified Bodies and Certificates (NB/CRF)
- Market Surveillance (MSU)
- Post-Market Surveillance and Vigilance (PMSV)
- Clinical Investigation & Performance Studies (CI/PS)
Four Modules Becoming Mandatory May 28, 2026
Commission Decision (EU) 2025/2371 confirms that the following four modules meet functional specifications and become mandatory on May 28, 2026:
Actor Registration: All manufacturers, authorised representatives, and importers must register to obtain a Single Registration Number (SRN). Without an SRN, you cannot register devices or upload certificates.
UDI/Device Registration: Manufacturers must enter every device sold in the EU market using the Unique Device Identification system. New devices launched from May 2026 must be registered before market placement. Legacy devices placed on the market before May 28, 2026 must be registered by November 28, 2026.
Notified Bodies and Certificates: All MDR/IVDR certificates issued after May 28, 2026 must be registered immediately. Pre-existing certificates must be uploaded by May 28, 2027.
Market Surveillance: Competent authorities will use this module to coordinate inspections and enforcement actions across member states.
Documents That Require Translation for EUDAMED Compliance
1: Instructions for Use (IFU)
MDR Article 10(11) and IVDR Article 10(10) require manufacturers to translate IFUs into the official language(s) of each member state where devices are marketed. Member states determine which languages are required.
IFUs must be clear, understandable, and appropriate for the intended user. For lay-use devices, translations must account for patient comprehension. Professional-use devices may have more flexibility in some member states.
2: Labels and Packaging
Essential safety information on labels requires accurate translation with standardized symbols and terminology. MDR Annex I Section 23 and IVDR Annex I Section 20 set out labeling requirements.
Member states determine language requirements for labels. Some countries require full translation, while others accept English for professional-use devices. Patient-facing devices almost always require local language translation.
3: Technical Documentation
Device descriptions, specifications, and risk management documents need translation for regulatory submissions. Technical documentation supports conformity assessment by notified bodies and competent authorities.
Translation requirements vary by document type and intended use. Manufacturers should verify requirements with their notified body and target member states.
4: Certificates and Declarations of Conformity
Notified Body certificates and EC/EU Declarations of Conformity require translation for EUDAMED registration. MDR Article 19(1) and IVDR Article 17(1) specify that declarations must be available in languages determined by member states where devices are placed on the market.
5: Summary of Safety and Clinical Performance (SSCP)
MDR Article 32 requires SSCPs for all Class III and implantable devices. IVDR Article 29 requires Summaries of Safety and Performance (SSP) for Class C and D devices.
MDCG guidance recommends translating SSCPs into languages accepted in member states where the device will be sold. These documents must be clear to intended users and, when relevant, to patients.
6: UDI and Device Registration Data
Product information and nomenclature in the UDI module require multilingual database entries. Device descriptions, intended purposes, and product specifications must be entered in formats that support member state language requirements.
EUDAMED has both a restricted site for regulatory authorities and a public site where patients and healthcare professionals access device information. Public-facing data must be accessible in appropriate languages.
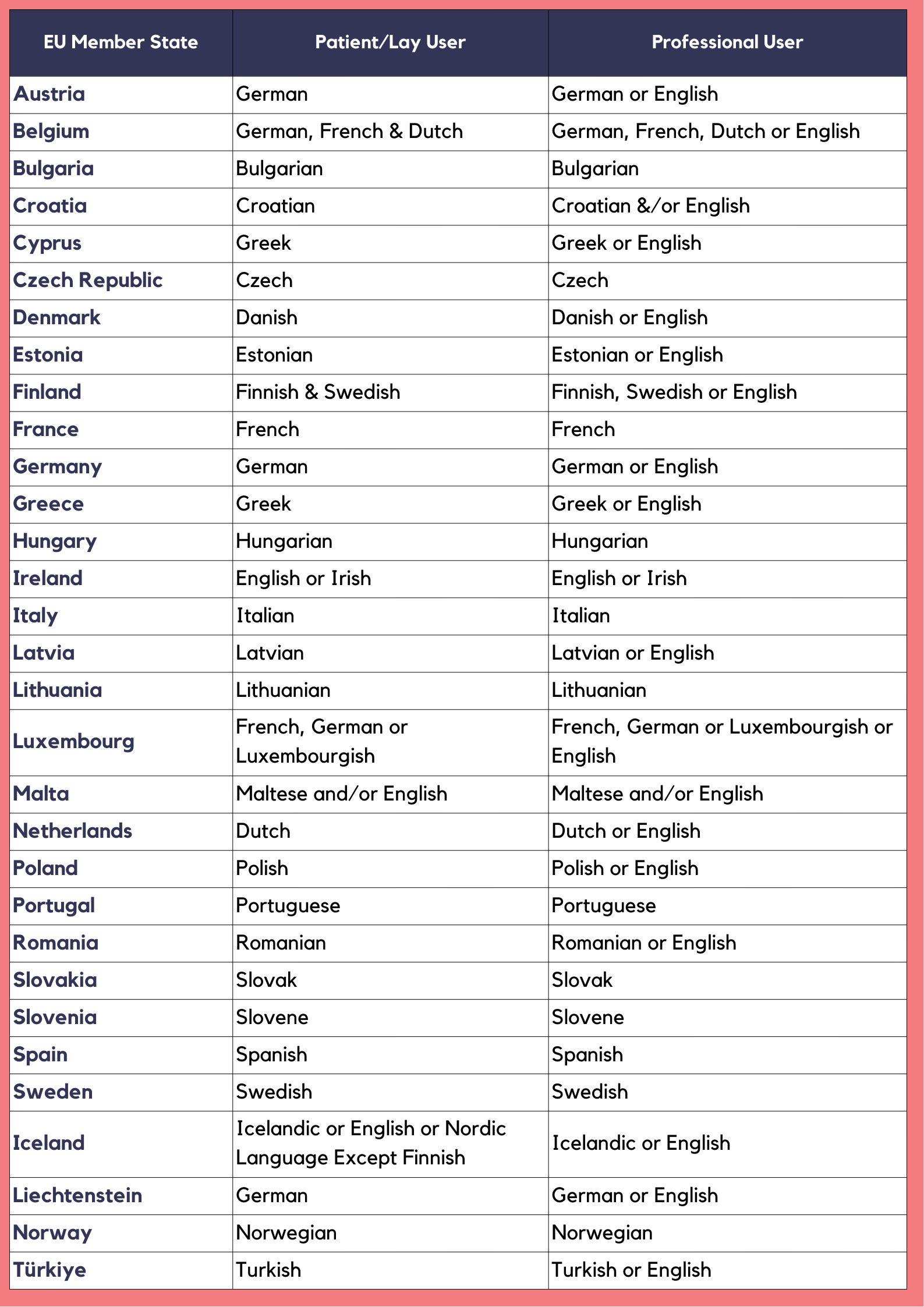
Language Requirements Across the 27 EU Member States
1: The 24 Official EU Languages
The European Union has 24 official languages as defined in Regulation No 1:
Bulgarian, Croatian, Czech, Danish, Dutch, English, Estonian, Finnish, French, German, Greek, Hungarian, Irish, Italian, Latvian, Lithuanian, Maltese, Polish, Portuguese, Romanian, Slovak, Slovenian, Spanish, and Swedish.
English remains an official EU language despite Brexit, as it is an official language in Ireland and Malta.
Several member states have multiple official languages. Belgium recognizes Dutch, French, and German. Luxembourg uses French and German. Malta uses Maltese and English. Ireland uses Irish and English.
2: Language Requirements by Module
Actor Registration: Registration forms and organizational data must be submitted according to EUDAMED specifications. Core registration information is typically in English, but supporting documentation may require member state languages.
UDI/Device Registration: Product descriptions, intended purposes, and device nomenclature must support multilingual access. Public-facing information must be available in the languages required by target markets.
Notified Bodies and Certificates: Certificates must be registered in EUDAMED and made available according to MDR/IVDR language requirements. Member states determine translation obligations for certificates.
Market Surveillance: Field Safety Notices and corrective action communications must be translated into the official EU languages required by countries where the device is available, as specified in MDR Article 89(8) and IVDR Article 84(8).
3: Country-Specific Considerations
Language requirements vary significantly by member state.
Some examples:
France: Labels and IFUs must be in French. Software interfaces for professional users can be in French or English, based on user needs and safety considerations.
Germany: Labels and IFUs must be in German. Some flexibility exists for professional-use devices.
Belgium: Requires Dutch, French, or German, depending on the region where the device is distributed.
Spain: Requires Spanish for most devices. Regional languages like Catalan may apply in specific regions.
The European Commission published official tables outlining language requirements by member states for MDR and IVDR. Manufacturers should consult these tables and verify requirements with target markets.
Looking for Medical Device Translation services?
Impact of the November 2025 Mandatory Compliance Decision on Translation Timelines
1: Key Dates and Deadlines
- November 27, 2025: Commission Decision (EU) 2025/2371 published in the Official Journal, confirming functionality of four EUDAMED modules and starting the six-month transition period.
- May 28, 2026: Mandatory use begins for Actor Registration, UDI/Device Registration, Notified Bodies & Certificates, and Market Surveillance modules. New devices must be registered before market placement.
- November 28, 2026: Legacy devices (placed on the market before May 28, 2026) must be registered in EUDAMED.
- May 28, 2027: Certificates issued before May 28, 2026, must be uploaded to EUDAMED.
- Q4 2026 (estimated): Full functionality notice expected for the Vigilance module.
- Q2 2027 (estimated): Mandatory use of the Vigilance module, approximately six months after functionality notice.
2: Translation Timeline Planning
- Professional translation requires significant time. For a typical medical device manufacturer with a moderate portfolio:
- Single language translation: 2-4 weeks for IFU and labeling, depending on complexity and word count.
- Multiple language projects (10+ languages): 6-12 weeks for IFU translation across major EU languages, including review cycles.
- Technical documentation translation: 4-8 weeks for device descriptions, specifications, and risk management files.
- Quality assurance and review: Additional 2-4 weeks for linguistic review, subject matter expert validation, and back-translation where required.
- Manufacturers with extensive portfolios or targeting all 27 member states should plan for 3-6 months of translation work to ensure May 2026 readiness.
3: Phased Approach to Translation
- Prioritize markets by revenue and strategic importance. Begin with major markets (Germany, France, Italy, Spain, UK-style English markets) before expanding to smaller member states.
- Leverage translation memory systems to maintain consistency across documents and reduce costs. Translation memory stores previously translated segments and reuses them in new projects, ensuring terminology consistency.
- Build a centralized terminology database covering product-specific terms, regulatory language, and standardized symbols. This ensures consistency across all languages and reduces translation time.
- Phase translation projects by risk class. Class III and implantable devices requiring SSCPs need earlier attention due to regulatory timelines. Class I devices may follow in subsequent phases.
Quality Standards and Best Practices for Regulatory-Compliant Translations
1: ISO Certification Requirements
Medical device translation is a regulated activity under quality management systems. Two ISO standards are critical:
ISO 17100:2015: Specifies requirements for translation service providers, covering translator competence, translation process, quality assurance, and project management. This standard ensures professional translation quality.
ISO 13485:2016: Defines quality management system requirements for medical device organizations. While this standard doesn’t specifically address translation, translation processes must integrate with your overall quality management system.
Translation service providers working with medical device manufacturers should hold ISO 17100 certification. Manufacturers should verify this certification before engaging translation partners.
2: Best Practices for Medical Device Translation
Use native expert translators: Translators must be native speakers of the target language with subject matter expertise in medical devices. General translators cannot adequately handle technical terminology and regulatory requirements.
Implement translation memory systems: Translation memory (TM) tools store previously translated segments and reuse them in new projects. This ensures consistency, reduces costs, and accelerates translation timelines.
Build terminology databases: Create glossaries of product-specific terms, regulatory language, and technical terminology. Translators use these databases to maintain consistency across all documents and languages.
Establish quality assurance processes: Implement multi-stage review, including linguistic review by a second translator, subject matter expert validation of technical accuracy, and final proofreading. For critical documents, consider back-translation to verify meaning preservation.
Maintain translation records: Document which language versions were provided to which markets. Keep evidence of translation validation for notified body and competent authority audits.
Consider user comprehension: Translations must be clear and understandable to the intended user in the target language. For lay-use devices, consider cognitive debriefing with actual users to verify comprehension.
3: Common Translation Pitfalls to Avoid
- Using machine translation alone: Machine translation tools cannot replace professional translators for regulatory documents. While they can assist in preliminary drafts, final translations require human expertise and validation.
- Inconsistent terminology: Using different terms for the same concept across documents or languages creates confusion and regulatory risk. Implement terminology management from the start.
- Underestimating timelines: Rushing translation projects increases error risk. Plan adequate time for translation, review, and quality assurance.
- Failing to integrate translation with QMS: Translation must be a controlled process within your quality management system, not an administrative afterthought. Document procedures, validation methods, and record-keeping.
- Overlooking updates: When you update source documents, remember to update all translated versions. Implement change control processes that trigger translation updates.
How Milestone Localization Can Help
Milestone Localization specializes in medical device translation services compliant with MDR, IVDR, and EUDAMED requirements.
ISO certifications: Milestone holds ISO 9001:2015 for quality management system, ISO 17100:2015 certification for translation services, and works within ISO 13485:2016 quality management frameworks.
Medical device expertise: The team includes translators with extensive experience in medical device documentation, regulatory submissions, and technical terminology.
Quality processes: Multi-stage review processes ensure accuracy, consistency, and regulatory compliance. Translation memory systems and terminology databases maintain quality across projects.
Proven track record: Milestone has supported numerous medical device manufacturers through MDR/IVDR transitions and EUDAMED registration, delivering translations that meet regulatory standards.
Contact Milestone Localization to discuss your EUDAMED translation needs and develop a comprehensive strategy for the May 2026 deadline.
Conclusion
EUDAMED becomes mandatory on May 28, 2026. Manufacturers have exactly six months to prepare.
Without compliant translations, manufacturers cannot register devices or maintain EU market access. Start translation planning immediately by assessing your device portfolio, identifying target markets, and engaging qualified translation partners.
Are You Looking for Certified Medical device Translation services?
FAQs
When does EUDAMED become mandatory for manufacturers?
EUDAMED becomes mandatory for manufacturers starting May 28, 2026, following the European Commission’s functionality notice and six-month transition period. From that date, manufacturers must use EUDAMED for registration activities across the four mandatory modules.
Which EUDAMED modules are mandatory from May 28, 2026?
From May 28, 2026, manufacturers must use EUDAMED for:
- Actor Registration (ACT)
- UDI/Device Registration (UDI/DEV)
- Notified Bodies and Certificates (NB/CRF)
- Market Surveillance (MSU)
Other modules (such as Vigilance and Clinical Investigations/Performance Studies) follow later as they reach full functionality.
What documents require translation for EUDAMED compliance?
Manufacturers should plan translations for key regulatory and user-facing content, including:
- Instructions for Use (IFU)
- Labels and packaging content
- Technical documentation (selected sections depending on submission needs)
- EU Declarations of Conformity
- Notified Body certificates
- SSCP/SSP summaries (where applicable)
- UDI and device registration information shown in EUDAMED
Do manufacturers need translations in every EU language?
No. Manufacturers must provide translations in the official language(s) of each EU member state where the device is marketed. If you sell in 5 countries, you translate for those markets – not all 24 EU official languages.
How early should manufacturers start translation planning for the May 2026 deadline?
Manufacturers should start as early as possible, since regulatory translation involves review cycles and quality controls. A realistic plan is:
- 2–4 weeks for single-language IFU/label translation
- 6–12 weeks for 10+ EU languages (including review cycles)
- 3–6 months for larger portfolios, SSCPs, and technical documentation with SME validation
Starting early also allows you to build terminology and translation memory for faster future updates.