Certified SMPC Translation Services
ISO 17100-compliant SMPC translations in 70 + languages, helping pharmaceutical companies navigate international regulatory approvals.
Translations Trusted by 400+ Global Companies
What is SMPC?
The Summary of Product Characteristics (SmPC) is a legal document approved as part of the marketing authorization of a medicinal product. It provides healthcare professionals with comprehensive and authoritative information on the safe and effective use of the medicine, including details on its formulation, approved indications, dosage, contraindications, and potential side effects.
Throughout the product’s lifecycle, the SmPC is continuously reviewed and updated as new clinical or safety data emerge, ensuring that healthcare providers have access to the information on how to use the medicine safely and effectively
What is the structure of an SMPC?
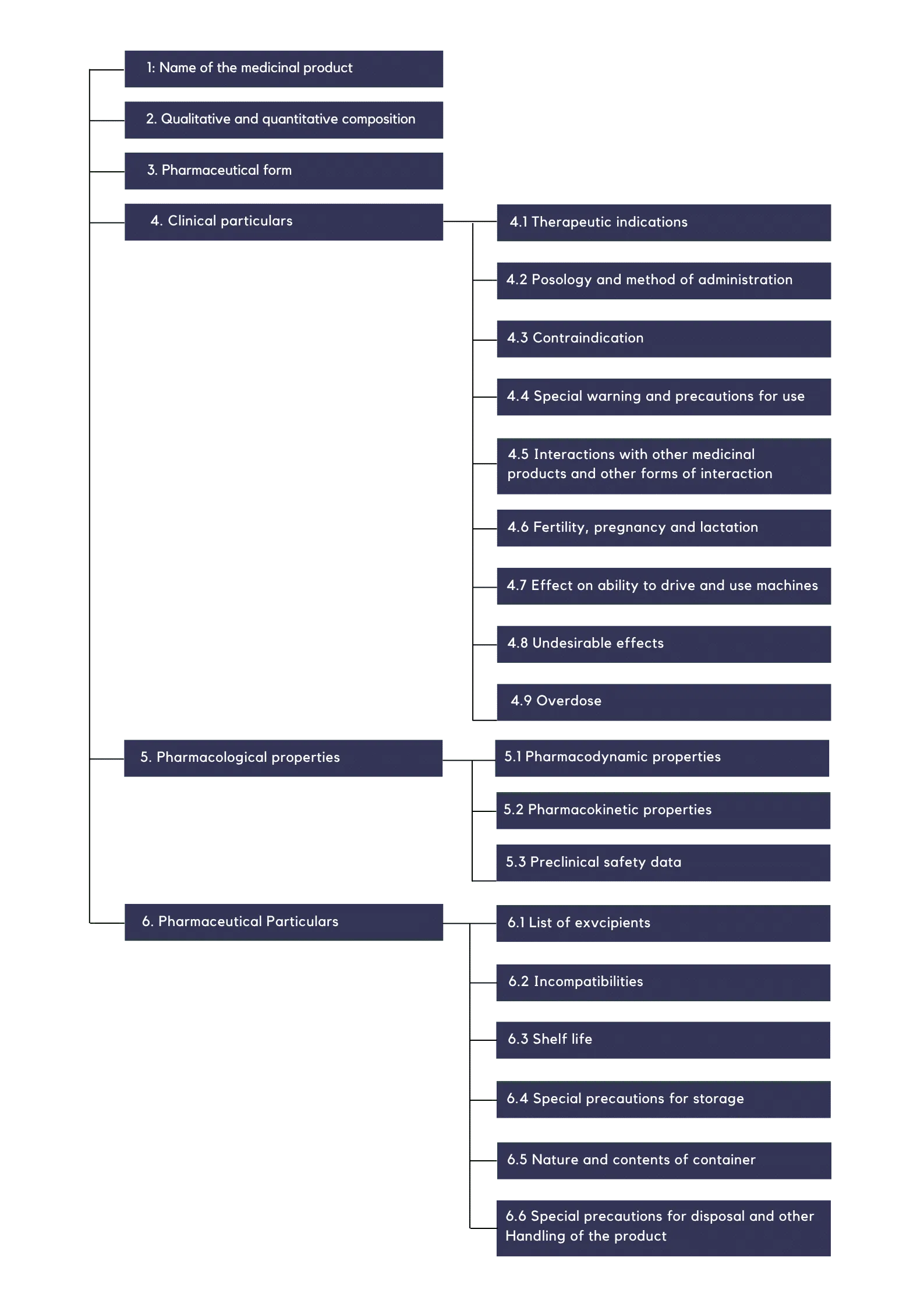
The Summary of Product Characteristics (SmPC) is organized according to a standardized structure mandated by regulatory authorities to ensure uniformity and clarity. This format provides healthcare professionals with comprehensive and systematically arranged information about a medicinal product, facilitating its safe and effective use.
The SmPC is divided into 6 key sections that cover all critical aspects of the medicine and they are as follows:
1: Name of the Product – Identifies the official name of the medicine.
2: Composition – Details the active ingredients and their concentrations.
3: Pharmaceutical Form – Describes the physical form in which the product is administered (e.g., tablet, injection).
4: Clinical Particulars – Covers essential clinical information such as approved uses, dosage recommendations, and safety precautions.
5: Pharmacological Properties – Explains how the medicine works in relation to its intended use, including potential side effects and interactions.
6: Pharmaceutical Particulars – Provides regulatory and technical details related to the formulation, storage, and packaging of the product.
Our Professional SMPC translation services
Ensuring the accuracy and clarity of the Summary of Product Characteristics (SmPC) is essential, as it serves as the primary reference for healthcare professionals regarding the safe and effective use of medicinal products. Translation of SmPCs plays a vital role in safeguarding patient safety, supporting regulatory compliance, and facilitating the proper use of medicines across diverse linguistic and cultural markets.
Our professional SmPC translation services are specifically tailored to meet the stringent demands of this essential document. With a dedicated team of medical translators and regulatory experts, we help pharmaceutical companies ensure their SmPCs meet global compliance standards, support clear communication with healthcare professionals, and facilitate the safe, effective use of their medicines across international markets.
We ensure that every translation strictly follows the QRD (Quality Review of Documents) template, preserving the standardized structure required by regulatory authorities. We also incorporate EDQM (European Directorate for the Quality of Medicines) standard terms and adhere to EMA (European Medicines Agency) terminology standards to maintain consistency, accuracy, and compliance across all target languages.
Our SMPC Translation Services
QRD template compliance
The QRD template, developed by the European Medicines Agency (EMA), standardizes the structure and content of SmPCs to ensure clarity, consistency, and regulatory compliance.
Our translation services strictly follow this format, preserving the required headings and phrasing across all language versions. By adhering to the QRD template, we help ensure that translated SmPCs are clear, uniform, and easy to navigate supporting regulatory approval and effective communication with healthcare professionals.

EDQM Standard Terms
The EDQM (European Directorate for the Quality of Medicines & HealthCare) Standard Terms are a comprehensive set of harmonized pharmaceutical terminology used across Europe to ensure consistency and clarity in medicinal product information.
We incorporate these terms into our SmPC translations to maintain precision, support regulatory compliance, and ensure clear communication for healthcare professionals and authorities.
EMA Terminology Standards
EMA Terminology Standards ensure consistent and accurate language across all pharmaceutical documents in the EU, reducing ambiguity and improving communication. We apply these standards in all our SmPC translations to ensure regulatory alignment, facilitate smoother approval processes, and provide healthcare professionals with accurate product information.
Why Choose Our SMPC Translation Services?
Strict non-disclosure policies to maintain the confidentiality of the SMPC documents

Expert Linguists with regulatory knowledge

Fast Turnaround Time

End-to-End Support
What to keep in mind when choosing your SMPC translation service provider
Certification of the translation agency
Look for ISO 17100 and ISO 9001 certified agencies. These certifications demonstrate that the agency has robust quality management systems in place to ensure accurate and high-quality SmPC translations that meet stringent regulatory requirements.
Native Translators
Ensure the agency works exclusively with native-speaking translators who have in-depth knowledge of pharmaceutical regulations, clinical terminology, and SmPC-specific content. This guarantees translations that are both linguistically and contextually accurate.
Confidentiality
SmPCs contain sensitive regulatory and proprietary information. Choose a provider that implements strict confidentiality protocols, including secure file handling, NDAs, and robust data protection measures throughout the translation process.
Industry expertise
Work with an agency that has extensive experience in pharmaceutical regulatory translation, particularly in SmPCs. Familiarity with EMA guidelines, QRD templates, and drug safety documentation is essential for accuracy and compliance.
Quality assurance process
For SmPC translations, choose a service that includes both translation and proofreading (T+P). Given the complexity of medical terminology, a rigorous quality assurance process is essential to ensure accuracy, consistency, and clarity.
Globally Recognized Translation Certificates
Ensure the agency provides certified translations accepted by international regulatory authorities and healthcare organizations. These certificates adds credibility to your SmPC submissions and support regulatory compliance across multiple markets.
Certified SMPC Translation Services
Accurate and certified translation of the Summary of Product Characteristics (SmPC) is necessary to ensure patient safety, regulatory compliance, and clear communication of essential product information across global markets.
Our team of SmPC translators are fluent in both source and target language and possess in-depth expertise in pharmaceutical terminology, regulatory guidelines, and clinical pharmacology relevant to SmPC content.
We deliver your translated SmPC documents in the required format, accompanied by a signed certificate of translation when requested, making them ready for submission to regulatory authorities and healthcare stakeholders.
Our certified SmPC translations are recognized and accepted worldwide by regulatory agencies, pharmaceutical companies, and healthcare organizations. Certificates are digitally signed and provided promptly, ensuring acceptance in international regulatory submissions.
What is the need for SMPC translation services
Regulatory Compliance
Many regulatory authorities mandate that the SmPC be provided in the local language as a prerequisite for marketing authorization. Translation ensures legal compliance and prevents delays in product authorization.
Improves Communication with Healthcare Professionals
Translating SmPCs into local languages improves understanding among healthcare professionals thereby supporting informed clinical decisions.
Facilitates Global Distribution
Translating the SmPC into multiple languages allows pharmaceutical companies to expand internationally by making product information accessible to healthcare professionals worldwide.
Essential for Pharmacovigilance Activities
Accurate SmPC translations clearly convey safety details, supporting effective monitoring and reporting of adverse reactions.
Ensures Patient Safety
The SmPC outlines key details like indications, dosage, and side effects. Accurate translation of this document ensures that healthcare professionals can use the medicine safely and effectively.
Our SMPC Translation Process

Accurate SmPC translation is essential for effective global communication, enabling pharmaceutical companies and regulatory authorities to convey critical product information clearly and consistently across languages. Here’s our process for delivering clear, accurate, and multilingual SmPC translation services:
1: We begin by thoroughly reviewing your SmPC translation requirements and the source documents. This enables us to assign native translators with expertise in regulatory affairs, clinical pharmacology, and pharmaceutical terminology relevant to your product.
2: Based on your project needs, we develop a customized terminology list and style guide. We incorporate EMA-approved terms, EDQM standard terminology, and ensure alignment with the QRD template to guarantee compliance and consistency throughout the translation.
3: Our project manager assigns the document to an experienced medical translator with subject matter expertise. They accurately translate all sections of the document, such as indications, dosage, contraindications, and pharmacological, while preserving the tone and intent of the original content.
4: After translation, the SmPC undergoes detailed editing and proofreading by subject matter experts. A final quality assurance check verifies accuracy and compliance with regulatory requirements and target market expectations.
- : We provide the translated SmPC for your review. Any feedback or preferred changes are addressed promptly and carefully, ensuring the final version reflects your expectations and regulatory needs.
6 After incorporating all feedback and completing a final review, we deliver the final file in your preferred format. Upon request, we also provide translation certificates to support submissions to regulatory authorities and stakeholders worldwide.

Certified Translations

ISO 17100:2015 & 9001:2015 Certified Agency

70+ Languages
98% OF OUR CLIENTS SAY THEY WOULD RECOMMEND OUR SERVICES TO A COLLEAGUE
"As a medical device company, we transitioned to EUMDR compliance in 2022 and needed translation on our IFUs in 22 international languages. We found Milestone Localization was best suited to do this work for us. Since then they have been our regular translation service provider with 100% on-time delivery of documentation."

“Exceptional service. Quick. Exceeded my goals by a mile. Would definitely choose to work with them again.”

"We delighted to share our positive experience with Milestone Localization. Over the course of several projects, their translation services have proven invaluable to our team. Milestone Localization consistently demonstrates a high level of professionalism, and their commitment to delivering accurate and timely services is commendable. The team's expertise shines through in every project they handle. Their skilled translators consistently provide top-notch results, making them trusted partner."

Order Your SMPC Translations Here
Get a free consultation on any questions you have related to translation & localization services.
Fill out the form and our team of experts will get in touch with you soon.
FAQS ON SMPC TRANSLATION SERVICES
What is a Summary of Product Characteristics (SmPC)?
The SmPC is a legal document that provides detailed information about a medicinal product’s properties, approved uses, dosage, contraindications, side effects, and precautions. It serves as a key reference for healthcare professionals.
Why is accurate SmPC translation important?
Accurate translation ensures that critical drug information is clearly and consistently communicated to healthcare providers across different countries, supporting patient safety and regulatory compliance.
Which regulatory standards must SmPC translations comply with?
SmPC translations must comply with guidelines such as the EMA’s QRD template and terminology standards, as well as local regulatory requirements in each target market.
Who can perform the SmPC translation?
Professional translators with expertise in pharmaceutical terminology, regulatory affairs, and clinical pharmacology can perform SmPC translations.
How do you ensure consistency in terminology across translations?
We develop customized terminology lists and style guides based on EMA and EDQM standards to maintain consistent use of medical and regulatory terms throughout the translation.
Do you provide certified translation for SMPC?
Yes, certified translations can be provided, accompanied by signed certificates that are accepted by regulatory authorities worldwide for submission and approval purposes.
How do you handle confidentiality and data security?
We follow strict confidentiality protocols, including secure file transfers, non-disclosure agreements (NDAs), and data protection measures to safeguard sensitive proprietary and regulatory information.
What languages do you translate SmPCs into?
We offer SmPC translation services in a wide range of languages to support pharmaceutical companies in accessing global markets.
How long does the SmPC translation process take?
The timeline depends on the document length, complexity, and language combination. We provide tailored project schedules and ensure timely delivery without compromising quality.