Medical Device Translation Services
Our team of highly specialised linguists provide accurate and compliant translations for medical device IFUs, product manuals, labels and patents.
Partner with our experienced team to translate your medical device documents in 70+ global languages.
Medical Device Translation Services For All Types of Devices
Our linguists have extensive experience in translating medical devices. We ensure to identify the best translators who can meet your specific requirements and provide precise and reliable translations.
Diagnostic Devices
Monitoring Devices
Prosthetics
Endoscopy Devices
Imaging Devices
Implants
Dental Devices
Orthopedic Devices
Surgical Instruments
Rehabilitation Devices
Respiratory Devices
Cardiovascular Devices
Trusted By Global Organizations In The Life Sciences Industry
We have earned the trust of leading organizations worldwide to handle their language translation needs. Our team of experts is committed to delivering accurate and effective translations that exceed your expectations.















Translations for Any Type Of Medical Device Document
Medical Device Packaging
Medical Device Labelling
Instructions For Use (IFUs)
Medical Device Operation Manuals
Safety Manuals
Clinical Evaluation Reports
Post Market Surveillance Reports
Standard Operating Procedures
Quality Assurance Documents
Patents
Importance of Medical Device Translation services
Medical device translation services are essential in the healthcare industry. Communication plays a key role in ensuring patient safety and complying with regulatory standards. Important documents such as user manuals, labels, packaging, instructions, and clinical trial papers, require precise and accurate translations.
The need and demand for precise and dependable translation services has increased due to the global nature of the healthcare industry. Milestone Localization is an ISO-certified company that focuses on providing accurate translation services for your medical devices. Our team of translators who extensive knowledge in the medical field ensure that the translations are accurate and also meet the regulatory standards
Our expertise helps minimize risks and enhance user satisfaction, allowing manufacturers to achieve international market goals while prioritizing patient safety.
Certified Medical Device Translation Services
Many regulatory bodies in the life science industry require translation certificates to ensure the accuracy and genuineness of translated documents.
Our translation certificates are accepted and acknowledged by regulatory authorities worldwide.
You can trust that our certified medical device translation services meet the necessary standards and will be recognized by relevant authorities, giving you complete confidence.
EU MDR-Compliant Medical Device Translations
The European Union Medical Device Regulation (EU MDR) aims to ensure that medical devices manufactured in or supplied to EU member countries meet high standards of safety and quality.
Manufacturers are responsible for complying with translation requirements and providing translations of all necessary documents in one or more official languages of EU member states. The translated materials must be accurate, and the source language must be clear.
The primary information required includes product details such as labeling, packaging, and Instructions for Use (IFU). Other information, such as user manuals and product safety documentation, are also subject to language requirements.
We work with a team of expert professionals to deliver precise translations that comply with EU MDR.
Translation Solutions for MDR & IVDR Compliance
Milestoneloc’s translation solutions are engineered to address the stringent new requirements of the EU Medical Device Regulation (MDR) EU 2017/745 and In Vitro Diagnostic Regulation (IVDR) EU 2017/746.
Our ISO 13485-certified processes rely on trained and certified human linguists specialized in medical device translations, ensuring 100% error-free and compliant output across all regulated content types — including Instructions for Use (IFUs), electronic IFUs (eIFUs), user manuals, SSCPs and SSPs, e-learning/training materials, software UI, and all other medical device documentation

Medical Device Software Localization
Today’s medical devices rely heavily on advanced software to perform complex functions. As a result, most medical device translation projects now include software localization—a process that involves extracting user interface (UI) strings, translating them into the target languages, and reintegrating them into the application.
Along with accurate UI translation, it’s essential to conduct linguistic quality assurance (LQA), as well as functional and visual testing, to ensure the software works flawlessly in every language.
At Milestone Localization, we follow proven localization workflows and agile processes to deliver high-quality, fast, and reliable results. Our approach aligns perfectly with modern, iterative software development, enabling us to localize all types of medical device software—mobile apps, embedded systems, and web-based platforms.
Instructions for Use (IFU) Translation
Regulatory authorities such as the EU require medical device manufacturers to provide translated Instructions for Use (IFU) in the local language. These documents outline the device’s purpose, target users, usage method, and safety information—whether it’s for doctors, healthcare professionals, or patients.
Accurate translation is critical to avoid misuse and ensure user safety. Besides technical precision, IFUs must also be fluent and compliant with local regulations.
Milestone Localization combines deep industry expertise, up-to-date regulatory knowledge, and advanced language tools to deliver IFU translations that are clear, consistent, and compliant. We are ISO 13485:2016 certified, which means we meet the highest quality standards specific to medical devices.


Medical Device Labelling Translation
To sell medical devices globally, product labels must also be translated in compliance with local regulations. These labels should clearly convey essential information about the device, including usage, safety, and warnings.
At Milestone Localization, we ensure that every translated label is accurate, clear, and aligned with regional regulatory requirements, helping you meet compliance and build trust in new markets.
Software as a Medical Device (SaMD) Translation
With the global rise of Software as a Medical Device (SaMD), the need for accurate and regulation-ready translation has never been higher—especially for European and Asian markets.
You’ve invested time and resources into developing your SaMD solution. Don’t let poor translation hold you back from going global.
At Milestone Localization, we specialize in localizing SaMD content, combining software expertise with in-depth knowledge of medical devices. Our services cover UI translation, linguistic and functionality testing, and localized user documentation to ensure your SaMD is market-ready in any language.

Our 6 Step Medical Device Translation Process
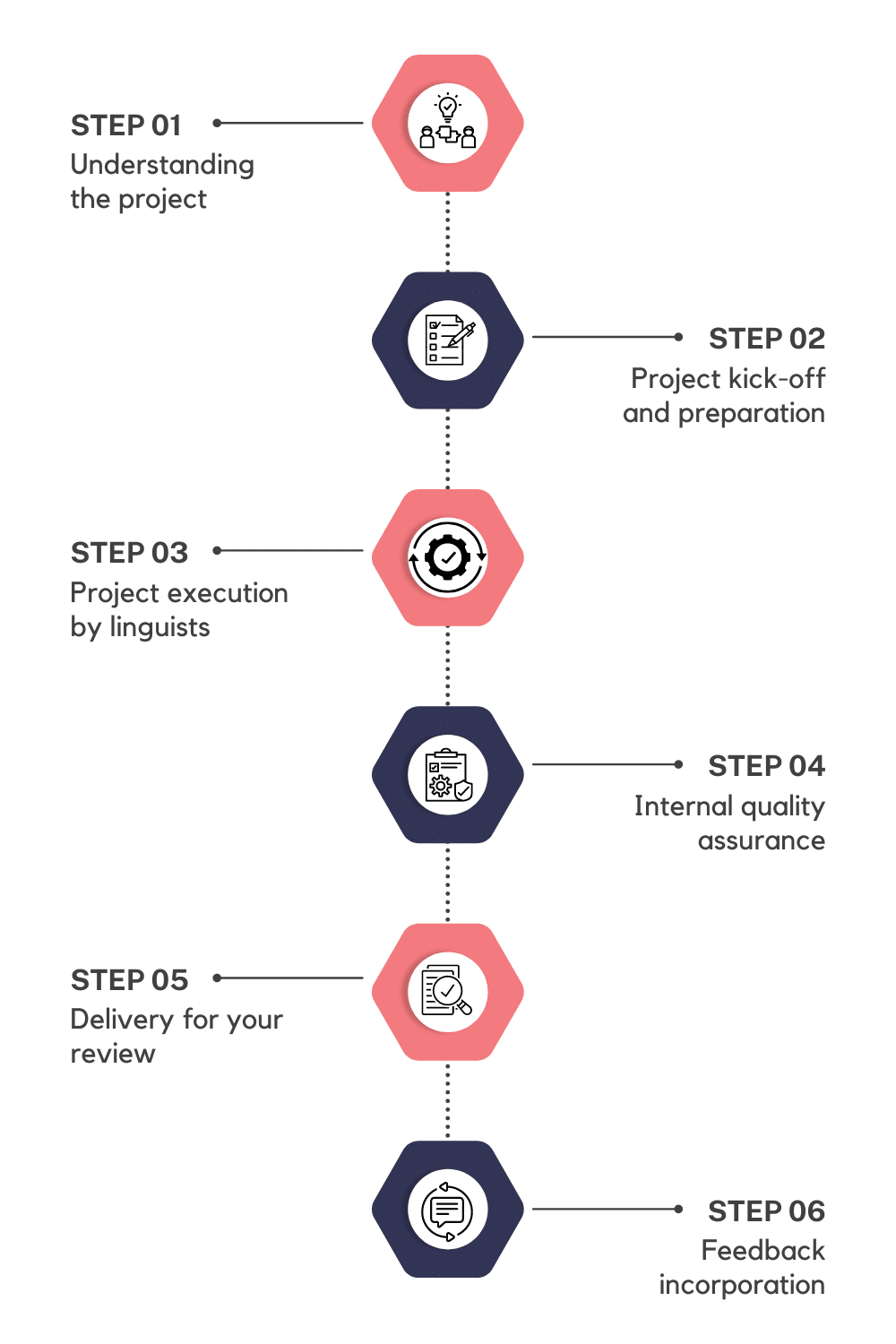
Understanding the scope of project, deadlines, client goals and file formats to prepare a custom project flow
Initiate the project and develop a custom translation style guide and glossary
Our team of native linguists, who possess extensive experience in translating medical device content, will undertake the translation and proofreading process
Internal quality assurance process will be conducted to validate the adherence to our QA checklist
Delivery of translated files for your review and feedback
Any feedback provided will be seamlessly integrated into the files and languages, ensuring the final deliverables are precise.
A GUIDE TO MEDICAL DEVICE TRANSLATION AS PER EU MDR
A free and comprehensive guide to help you seamlessly navigate the new language requirements as per the European Medical Device Regulation.

Why choose our medical device translation services?

ISO 17100:2015 and 9001:2015 certified agency
Serving 40+ top global companies in the life science industry

Network of 1200+ native linguists with subject matter expertise
Words translated for healthcare companies every year

Globally accepted translation certificates

Top Medical Translation Company in 2025
Our medical device translation services are available in 70+ languages
Spanish
Bulgarian
Croatian
German
Portuguese
Lithuanian
Estonian
Italian
French
Japanese
Chinese
Hindi
Testimonials
Hear From Our Clients In The Life Sciences Industry
"As a medical device company, we transitioned to EUMDR compliance in 2022 and needed translation on our IFUs in 22 international languages. We found Milestone Localization was best suited to do this work for us. Since then they have been our regular translation service provider with 100% on-time delivery of documentation."

“Exceptional service. Quick. Exceeded my goals by a mile. Would definitely choose to work with them again.”

"We delighted to share our positive experience with Milestone Localization. Over the course of several projects, their translation services have proven invaluable to our team. Milestone Localization consistently demonstrates a high level of professionalism, and their commitment to delivering accurate and timely services is commendable. The team's expertise shines through in every project they handle. Their skilled translators consistently provide top-notch results, making them trusted partner."

"Our experience with Milestone Localization has been highly satisfactory. They have met our expectations. One aspect we really appreciate is their prompt responsiveness. Whenever we've had questions or needed assistance, they have been quick to respond and provide support. This level of responsiveness has made working with them a breeze. In terms of quality, we've found their translations to be accurate and appropriate for our high needs. Moreover, we greatly appreciate their professionalism. Prior to starting each translation, they consistently ensure alignment with our requirements and meticulously check various aspects such as terminology lists and style guides. Their professionalism and prompt service is very much valued, and we can only recommend their services."

“The team at Milestone has been great to work with! They are responsive and provide quality translations that meet our project specifications in a timely manner. If you’re looking for translation services, we recommend checking them out!”

"Working with Milestone Localization has been an excellent experience for us. Their communication is clear, prompt and professional at every stage. The translations are not only accurate but are delivered on time, every time. It’s rare to find a company that combines quality with reliability so well. Highly recommended!"

Blogs About Medical device
Translation

Fastest Growing Medical Device Markets & Need For Translation
The global medical devices market has been growing rapidly in recent years, with new technologies & innovations driving growth in key regions worldwide. According to reports, the medical device market is expected to make a revenue of $595 billion in 2024, & projections suggest it will reach $886.80 billion by 2032.

Quality Assurance In Medical Device Translations
Medical device translation refers to the process of translating various types of documentation related to medical devices. This includes the translation of medical documents, such as user manuals, instructions for use (IFU), labels, packaging materials, software interfaces, various marketing materials, and many others.
IFU Translation : Importance, Requirements & Best Practices
Instructions For Use or IFUs serve as essential documents that provide important information about the proper use of products, devices, and equipment. . In this blog, we will explore the requirements for IFUs in the medical device industry, including IFU translation and how to approach it.
The Importance of eIFU Translation in Medical Device Compliance
In today’s digital age, eIFUs are crucial in the medical device industry. They enable real-time updates, better accessibility, and compliance with global regulations. However, accurate eIFU translation remains a complex challenge that affects patient safety, compliance, and global market access.
Get in touch
Our team is ready to help you with your translation needs
Other ways to reach us
FAQs ON MEDICAL DEVICE TRANSLATION SERVICES
How much do medical device translation services cost?
The medical device translation cost varies according to the word count, type of content, language pair, and turnaround time. Get in touch to get a free quotation.
How long does medical device translation take?
The time taken to translate a document related to medical devices depends on the complexity, length, language pair and overall nature of the document. We can provide you with a timeline after an analysis of your project.
Can you handle the translation of medical device documentation according to MDR guidelines?
Yes, we work with a team of subject matter experts and highly qualified medical professionals for our medical translation projects.
Our team is well aware of MDR’s intricacies and is well-versed in the terminology, documentation requirements, and translation standards demanded by MDR. We are also ISO 17100 certified and we provide a digitally signed certificate that can be used for regulatory purposes.
How do you ensure that the translations meet MDR requirements?
We have a comprehensive translation process, including proofreading, editing and quality assessment. Our highly qualified subject matter experts meticulously work on each step of the process to ensure that our translations meet MDR requirements.
We ensure that our translations align with MDR standards, helping our clients navigate regulatory challenges seamlessly.
Will translation certificates be provided for my translations? If yes, will regulatory authorities accept your translation certificates?
Yes, we provide translation certificates that certify the accuracy and completeness of our translations. As an ISO 17100-certified translation agency, our translation certificates are authentic and accepted by regulatory authorities worldwide.
How do you maintain the confidentiality of the data?
Maintaining confidentiality of the data is of utmost priority to us. We use the latest technology, strict non-disclosure agreements and conduct regular audits to ensure confidentiality and data security.






