Clinical Trial Translation Services
Our team of highly specialized linguists provides accurate translations for all your clinical trial documents
Get your clinical trial documents translated into 70+ languages on time.

Why Clinical Trial Translations
In today’s world, clinical trial translation services play a vital role in the healthcare industry due to the rapid advancements in medical research and the increasing complexity of healthcare. These services act as a bridge between research and clinical practice and help to translate scientific discoveries and findings into practical applications that can be used in clinical settings to improve patient outcomes.
The focus on patient-centered care has become increasingly important in recent years, and clinical translation services are essential in ensuring that research findings are translated into personalized care plans that are tailored to the unique needs of each patient. This personalized approach to care helps to improve patient outcomes and promote patient satisfaction.
Furthermore, the healthcare industry is subject to strict regulatory requirements and ethical considerations. Clinical trial translations help to ensure compliance with these standards, promoting patient safety and preserving the integrity of the research.
By working with regulatory agencies and ethics committees, clinical translation services can help to ensure that research findings are used in a responsible and ethical manner.
Our clinical trial document translation services
Quality of life (QoL) measures
Patient recruitment materials
Pharmacovigilance materials
Summary of product characteristics (SmPC)
Contracts & agreements
Site-facing materials
SAEs and SUSARs
Case report forms (CRFs)
Operational procedure guidelines
Clinical outcome assessments (COAs)
Clinical study report (CSR)
Patient-facing materials
IVR / IWR systems
Adjudication materials
Patient-reported outcomes (PROs)
Regulatory letters and applications
Informed consent forms (ICFs)
Patient information leaflets (PILs)
Clinical trial protocols
Product Labels
Training manuals
Pharmacological studies
Patents and other IP documents
Instructions for Use (IFUs)
Budget and fee documentation
Surgical instructions
Marketing materials
Regulatory Documents
Why do you need Clinical Research translation services?
Clinical trials are essential research studies that test the safety, efficacy, and effectiveness of new medical treatments, drugs, or devices in human participants. These trials are often conducted across multiple countries and regions to gather diverse data and ensure that the findings are applicable to a broad population.
However, conducting clinical trials on a global scale presents significant challenges, especially when it comes to language and cultural differences because it is necessary to ensure that all participants, investigators, and regulatory authorities fully understand the information in their native language, while maintaining consistency and compliance with strict regulations.
Therefore, translating clinical trial documents accurately is an essential step in the research process. By facilitating clear communication across different languages and cultures, these services are essential for the successful execution of global clinical trials.
Trusted By Global Organizations In The Life Sciences Industry
We have earned the trust of leading organizations worldwide to handle their language translation needs. Our team of experts is committed to delivering accurate and effective translations that exceed your expectations.













Certified Clinical Trial Translation Services
Translation certificates are often required by regulatory bodies in the life science industry to guarantee that translated documents are accurate and authentic.
We offer certified translations for a variety of documents to comply with this requirement.
Our translation certificates are recognized and accepted by regulatory authorities worldwide, providing you with complete assurance that our translations meet the necessary standards and will be confidently acknowledged by relevant authorities.
Common Technical Document Translation
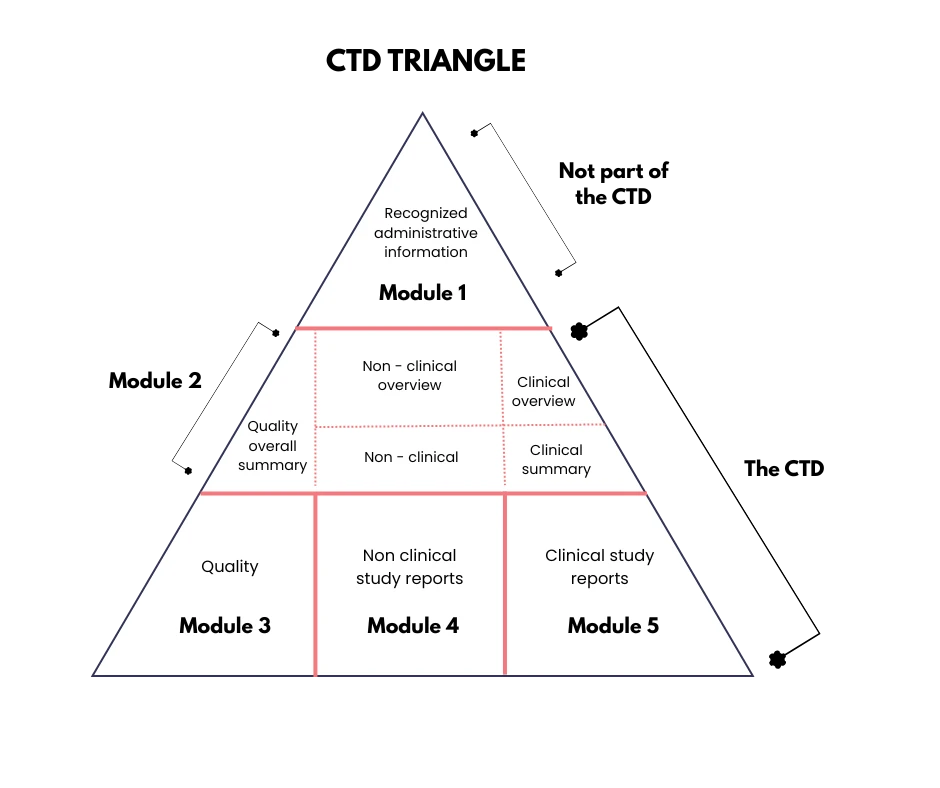
When registering medicines with international regulatory authorities such as the European Union, Japan, or China, accurate and professional translation of Common Technical Document (CTD) modules is necessary for compliance with harmonized electronic submission and global regulatory review standards.
The CTD, developed by the International Council for Harmonisation (ICH), standardizes the format for drug registration across regions, streamlining the review process. It consists of five key modules, all of which may require translation: Module 1 (administrative and prescribing information), Module 2 (summaries of quality, safety, and efficacy), Module 3 (Quality – M4Q), Module 4 (Safety – M4S), and Module 5 (Efficacy – M4E).
These documents include pharmaceutical quality data, preclinical toxicology reports, and clinical trial results. Ensuring precise, contextually accurate translations of these technical materials is essential to support regulatory approvals and global market access.
Why choose our clinical trial translation services?
Confidentiality and Data Security
Specialized Medical Expertise

Fast Turnaround Time

Compliant and Certified
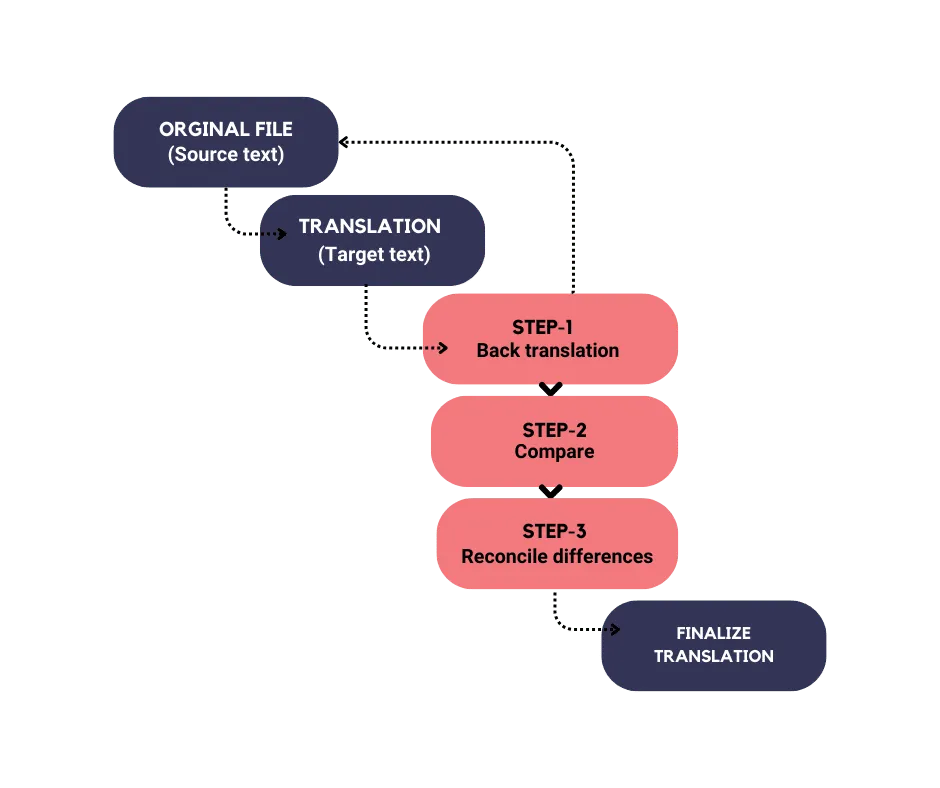
Back Translation
Back translation is an important tool in the field of clinical translation. It helps to ensure accuracy and consistency throughout the translation process by translating a document back into the original language using a different translator.
By comparing the original document with the back-translated version, back translation helps identify any inconsistencies in terminology and ensures quality assurance.
In addition, it can assist with the validation of translations. Regulatory bodies may require back translation as part of the validation process for translated documents.
Overall, back translation plays a crucial role in ensuring accurate and culturally sensitive communication in clinical translation.
Terminology management in clinical translations
Terminology management plays an important role in the translation of clinical trials, ensuring accuracy, consistency, and regulatory compliance. A key aspect involves the development of comprehensive glossaries and databases containing terminology specific to the clinical trial domain.
It consists of medical terms, drug names, abbreviations, and technical terminology related to the study. Official translations of medical terms and internationally recognized dictionaries are also utilized in the process.
We also leverage technology such as translation memory tools and terminology management software to facilitate efficient management of glossaries, ensuring consistency throughout translations.
Additionally, we adhere to regulatory requirements for terminology usage to ensure compliance with international standards.
What is the need for clinical translation services?
Global Trial Expansion
Clinical trial translation services are essential for adapting study materials to diverse languages and cultural contexts, enabling trials to be conducted across multiple countries. Accurate translation allows sponsors to access broader patient populations while ensuring compliance with local regulations. This facilitates the efficient and ethical conduct of global clinical research.
Accurate Communication of Medical Information
Given the complexity of clinical trial data and protocols, translation is necessary to ensure clarity of medical terminology and procedures, thereby preserving the scientific accuracy and consistency required across trial sites and safeguarding the integrity and reliability of trial outcomes.
Patient Safety and Compliance
Accurate translation of informed consent forms, safety information, and procedural guidelines is essential to guarantee that participants fully understand the risks, benefits, and requirements of the trial, thereby protecting patient safety and adhering to stringent regulatory and ethical standards
Enhanced Patient Recruitment and Retention
Providing trial information in participants’ native languages fosters better comprehension and trust, significantly improving recruitment efficiency and participant retention throughout the study.
Consistency and Standardization Across Documentation
Professional translation services ensure uniformity and precision across all clinical trial documents including protocols, questionnaires, and regulatory submissions—facilitating regulatory approvals and maintaining the reliability and validity of trial outcomes.
How we work with Clinical Research Organizations (CROs)

Accurate medical & pharmaceutical translations are crucial for any CRO, as they need to comply with government regulations, laws, and industry standards.
Partnering with an ISO-certified language service provider is essential to ensure that the clinical trial translations are accurate and understandable in the native languages of the research teams and subjects.
Here’s how we ensure that our translations are accurate and of the highest quality:
1. Our team comprises expert linguists and subject matter specialists who provide accurate translations for a wide range of medical records.
2. We ensure the highest quality translations by working with translators having extensive knowledge and experience in the life science sector.
3. All translated documents are proofread, and edited and also go through quality analysis to ensure accuracy. Additionally, for clinical trial translations, back translation is also conducted.
5. We strive to ensure that our translations uphold the quality, accuracy, and authenticity of the original content.
6. We understand the importance of meeting tight deadlines and guarantee on-time delivery of translations.
Testimonials
Hear From Our Clients In The Life Sciences Industry
"As a medical device company, we transitioned to EUMDR compliance in 2022 and needed translation on our IFUs in 22 international languages. We found Milestone Localization was best suited to do this work for us. Since then they have been our regular translation service provider with 100% on-time delivery of documentation."

“Exceptional service. Quick. Exceeded my goals by a mile. Would definitely choose to work with them again.”

"We delighted to share our positive experience with Milestone Localization. Over the course of several projects, their translation services have proven invaluable to our team. Milestone Localization consistently demonstrates a high level of professionalism, and their commitment to delivering accurate and timely services is commendable. The team's expertise shines through in every project they handle. Their skilled translators consistently provide top-notch results, making them trusted partner."

"Our experience with Milestone Localization has been highly satisfactory. They have met our expectations. One aspect we really appreciate is their prompt responsiveness. Whenever we've had questions or needed assistance, they have been quick to respond and provide support. This level of responsiveness has made working with them a breeze. In terms of quality, we've found their translations to be accurate and appropriate for our high needs. Moreover, we greatly appreciate their professionalism. Prior to starting each translation, they consistently ensure alignment with our requirements and meticulously check various aspects such as terminology lists and style guides. Their professionalism and prompt service is very much valued, and we can only recommend their services."

“The team at Milestone has been great to work with! They are responsive and provide quality translations that meet our project specifications in a timely manner. If you’re looking for translation services, we recommend checking them out!”

Blogs About Translations for Clinical Trials

The Role of CROs in Managing Multilingual Clinical Trial Documentation
As the demand for multilingual clinical trials grows, so does the importance of accurate, culturally adapted translations. With clinical trials often spanning across multiple countries and involving participants from all over the world, medical translation is now the best tool for managing the increasingly international medical field.

Translating Clinical Trials: Global Implications and Responsibilities
Clinical trials are research-oriented studies conducted with human participants to evaluate the safety, efficacy, and potential side effects of new medical treatments, drugs, vaccines, or medical devices. These trials are essential in advancing medical science by testing new interventions before they can be approved for widespread clinical use.
ICF Translation: Importance, Requirements & Best Practices
Informed consent is a fundamental ethical principle in research that ensures that participants or the subjects of the study fully understand the nature of the study and voluntarily agree to participate. It involves providing individuals with comprehensive information about the research study, including its purpose, procedures, potential risks, and benefits.
Get in touch
Our team is ready to help you with your translation needs
Other ways to reach us
FAQS ON Clinical Trial translation services
What are clinical trial translation services?
Clinical trial translation services involve translating all documents related to clinical research, such as protocols, consent forms, and reports, into different languages to ensure clear communication with participants and regulatory bodies.
Which types of documents require translation in clinical trials?
Documents like informed consent forms, patient questionnaires, trial protocols, investigator brochures, case report forms, and regulatory submissions typically need translation.
Can you provide certified translations for regulatory submissions?
Yes, certified translations with formal attestations can be provided to meet regulatory agency requirements.
Do you utilize subject-matter experts?
Yes, all our language services for clinical trials are carried out by professional linguists with subject-matter expertise in life sciences, clinical research, regulatory affairs, and more.
The chosen linguist(s) will be a native speaker of the target language and will hold a degree or relevant qualification in the desired field.
How do you ensure the confidentiality of clinical trial documents?
We follow strict confidentiality protocols, including NDAs, secure data transfer systems, and ISO-certified information security management to protect sensitive client data.
What quality control processes do you use for clinical trial translations?
Our process includes multiple rounds of review, editing by subject matter experts, back-translation when needed, and adherence to industry standards like ISO 17100.
How do you maintain terminology consistency across large, multi-document projects?
We utilize translation memory tools and maintain customized glossaries and terminology databases tailored to each client and project.


